Innovation in Action: Top Companies Developing Breast Cancer Treatments
Breast cancer is a form of cancer that arises from breast cells. Breast cancer is more common in women, but men can develop it as well. This condition develops when the cells in the breast begin to grow uncontrollably, resulting in a tumor that can be seen on an X-ray or feels like a lump. If not treated, breast cancer can spread (metastasize) to other regions of the body. The prognosis varies by kind, stage, and other factors. Early identification and treatment are critical to increasing survival rates. Regular follow-up is required to assess for recurrence and manage any residual treatment effects. Breast cancer research is always improving, resulting in new treatments and better outcomes for patients. Early identification and individualized treatment strategies are critical for successfully controlling this disease.
The landscape of breast cancer treatment and diagnostics is constantly changing, with contributions from both traditional pharmaceutical companies and emerging biotech firms. Advances in genetic testing, targeted medicines, immunotherapy, and personalized medicine are opening the door to more effective and individualized treatment options, with the ultimate goal of improving outcomes for breast cancer patients.
Following are the Breast Cancer Drug manufacturers:
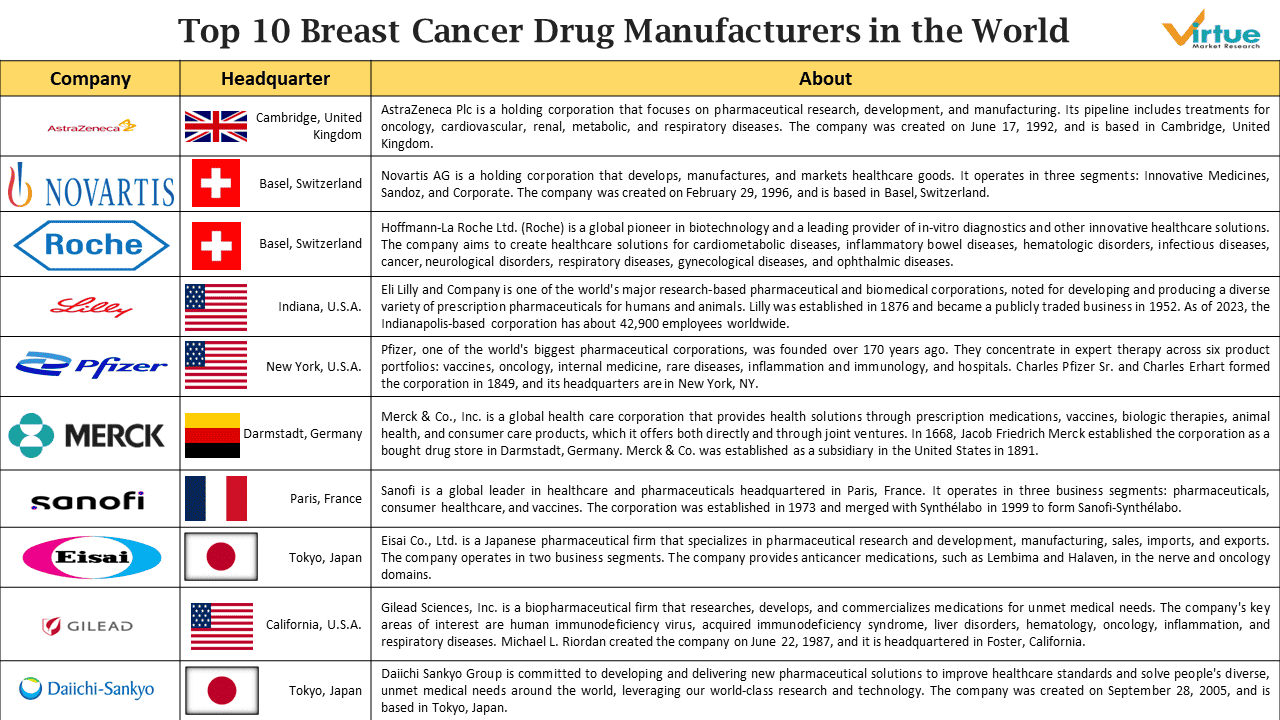
1. AstraZeneca PLC
AstraZeneca Plc is a holding corporation that focuses on pharmaceutical research, development, and manufacturing. Its pipeline includes treatments for oncology, cardiovascular, renal, metabolic, and respiratory diseases. The company was created on June 17, 1992, and is based in Cambridge, United Kingdom. In November 2023, Truqap in combination with Faslodex was approved In US for certain patients with advanced HR-positive breast cancer. Lynparza is approved in 97 countries for gBRCAm, HER2-negative early breast cancer (approved in the metastatic setting in 80 countries).
- Key Products: Faslodex (fulvestrant), Lynparza (olaparib), Truqap (capivasertib).
- Company Revenue: $45,811 Million.
2. Novartis
Novartis AG is a holding corporation that develops, manufactures, and markets healthcare goods. It operates in three segments: Innovative Medicines, Sandoz, and Corporate. Novartis Oncology and Novartis Pharmaceuticals are the two business areas that comprise the Innovative Medicines segment, which conducts research, development, manufacturing, distribution, and sales of patented pharmaceutical products. Sandoz develops, manufactures, and markets finished dosage form medications, as well as intermediate goods containing active pharmaceutical components. The Corporate section covers group management and central services. The company was created on February 29, 1996, and is based in Basel, Switzerland. Kisqali is approved in the US, the EU and other countries to treat advanced or metastatic hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) breast cancer.
- Key Products: Kisqali (ribociclib), Afinitor (everolimus).
- Company Revenue: $45,400 Million.
3. F. Hoffmann-La Roche Ltd
Hoffmann-La Roche Ltd. (Roche) is a global pioneer in biotechnology and a leading provider of in-vitro diagnostics and other innovative healthcare solutions. The company aims to create healthcare solutions for cardiometabolic diseases, inflammatory bowel diseases, hematologic disorders, infectious diseases, cancer, neurological disorders, respiratory diseases, gynecological diseases, and ophthalmic diseases. Sales of Perjeta grew by 1%, with the international region, particularly China and Brazil, being key growth driver. This was partly offset by a decline in sales in the US as a result of an adjustment related to US governmental plans. Excluding this adjustment, Perjeta sales in the US showed a growth of 1%. Sales of Perjeta in Europe were lower following the launch of Phesgo.
- Key Products: Herceptin (trastuzumab), Perjeta (pertuzumab), Kadcyla (ado-trastuzumab emtansine).
- Company Revenue: $69,752 Million.
4. Eli Lilly and Company
Eli Lilly and Company is one of the world's major research-based pharmaceutical and biomedical corporations, noted for developing and producing a diverse variety of prescription pharmaceuticals for humans and animals. Lilly was established in 1876 and became a publicly traded business in 1952. As of 2023, the Indianapolis-based corporation has about 42,900 employees worldwide. Revenue of Verzenio increased 52 percent in the U.S., driven by increased demand, and, to a lesser extent, higher realized prices. Revenue outside the U.S. increased 63 percent, driven by increased demand, partially offset by lower realized prices and the unfavourable impact of foreign exchange rates.
- Key Products: Verzenio (abemaciclib).
- Company Revenue: $34,124 Million.
5. Pfizer
Pfizer, one of the world's biggest pharmaceutical corporations, was founded over 170 years ago. They concentrate in expert therapy across six product portfolios: vaccines, oncology, internal medicine, rare diseases, inflammation and immunology, and hospitals. Charles Pfizer Sr. and Charles Erhart formed the corporation in 1849, and its headquarters are in New York, NY. Ibrance sale declines primarily driven by lower demand globally due to competitive pressure, lower clinical trial purchases internationally, and planned price decreases in certain international developed markets. Talzenna is approved in U.S. on October 2018, in EU on June 2019, and in Japan on January 2024.
- Key Products: Ibrance (palbociclib), Talzenna (talazoparib).
- Company Revenue: $58,496 Million.
6. Merck & Co.
Merck & Co., Inc. is a global health care corporation that provides health solutions through prescription medications, vaccines, biologic therapies, animal health, and consumer care products, which it offers both directly and through joint ventures. The Company's operations include pharmaceuticals, animal health, and consumer care. In 1668, Jacob Friedrich Merck established the corporation as a bought drug store in Darmstadt, Germany. Merck & Co. was established as a subsidiary in the United States in 1891.
- Key Products: Keytruda (pembrolizumab). In September 2022, MHLW approved Keytruda in combination with chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment after surgery for patients with hormone receptor-negative and HER2- negative breast cancer at high risk of recurrence.
- Company Revenue: $60,115 Million.
7. Sanofi
Sanofi is a global leader in healthcare and pharmaceuticals headquartered in Paris, France. It operates in three business segments: pharmaceuticals, consumer healthcare, and vaccines. The Pharmaceuticals business includes the commercial operations of the following worldwide franchises: specialized care, diabetes and cardiovascular, established prescription medicines and generics, and R&D and production activities. The Consumer Healthcare sector include the commercial operations for its Consumer Healthcare goods. The Vaccines section includes Sanofi Pasteur's commercial operations.
- Key Products: Taxotere (docetaxel).
- Company Revenue: $46,762 Million.
8. Eisai Co., Ltd.
Eisai Co., Ltd. is a Japanese pharmaceutical firm that specializes in pharmaceutical research and development, manufacturing, sales, imports, and exports. The company operates in two business segments. The Pharmaceutical segment is involved in the research, development, manufacture, and marketing of pharmaceuticals for medical purposes, generic drugs, and general medicines, among other things. The Others division is involved in license revenue and pharmaceutical raw material sales. The company also provides anticancer medications, such as Lembima and Halaven, in the nerve and oncology domains. Halaven is approved for use in the treatment of breast cancer in over 85 countries including Japan, the United States, in Europe, China and in Asia.
- Key Products: Halaven (Eribulin Mesylate).
- Company Revenue: $ 4,899 Million.
9. Gilead Sciences, Inc.
Gilead Sciences, Inc. is a biopharmaceutical firm that researches, develops, and commercializes medications for unmet medical needs. The company's key areas of interest are human immunodeficiency virus, acquired immunodeficiency syndrome, liver disorders, hematology, oncology, inflammation, and respiratory diseases. Michael L. Riordan created the company on June 22, 1987, and it is headquartered in Foster, California. FDA approved Trodelvy for the treatment of unresectable locally advanced or metastatic HR+/HER2- breast cancer who have received endocrine-based therapy and at least two additional systemic therapies in the metastatic setting. EC approved Trodelvy as monotherapy for the treatment of adult patients with unresectable or metastatic HR+/HER2- breast cancer who have received endocrine-based therapy and at least two additional systemic therapies in the advanced setting. Trodelvy product sales increased 56% to $1.1 billion in 2023, compared to 2022, primarily due to higher demand in new and existing geographies.
- Key Products: Trodelvy (Sacituzumab Govitecan-hziy).
- Company Revenue: $27,116 Million.
10. Daiichi Sankyo Company
Daiichi Sankyo Group is committed to developing and delivering new pharmaceutical solutions to improve healthcare standards and solve people's diverse, unmet medical needs around the world, leveraging our world-class research and technology. Daiichi Sankyo and its 15,000 employees worldwide build on a long tradition of invention and a solid pipeline of potential new medications to treat people. The company was created on September 28, 2005, and is based in Tokyo, Japan. Enhertu was approved in the EU and China as the first HER2-directed therapy for patients with HER2-low metastatic breast cancer based on the DESTINY-Breast04 Phase III trial. Enhertu is approved in more than 55 countries for HER2-positive metastatic breast cancer following one or more prior anti-HER2-based regimen. Also approved in more than 40 countries for HER2-low metastatic breast cancer following chemotherapy. Enhertu for treatment of HER2-positive breast cancer 2L was approved in US on May 2022, in EU on July 2022, and in Japan on November 2022. Enhertu for treatment of HER2 low breast cancer 2L was approved in US on August 2022, in EU on January 2023, and in Japan on March 2023. Enhertu launched in 35 countries and regions.
- Key Products: Enhertu (Trastuzumab Deruxtecan).
- Company Revenue: $8,442 Million.