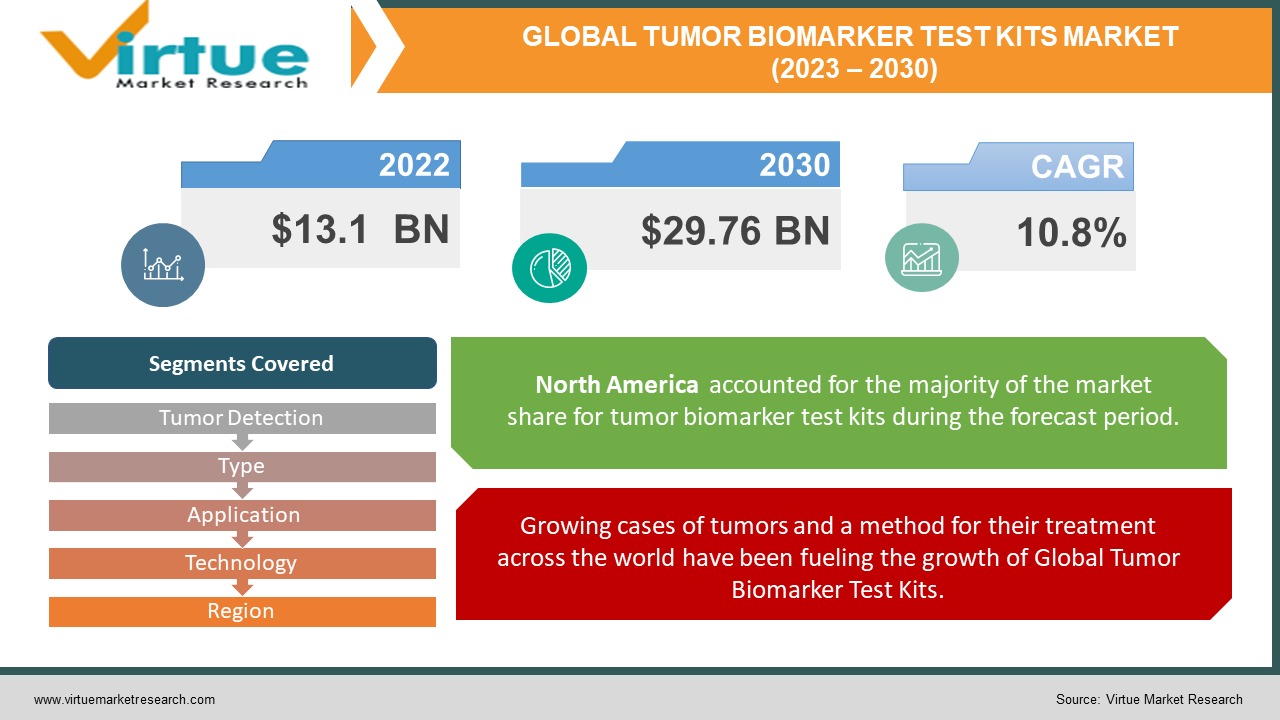
Tumor Biomarker Test Kits Market Size (2023 – 2030)
The global Tumor Biomarker Test Kits Market is estimated to be worth USD 13.1 Billion in 2022 and is projected to reach the value of USD 29.76 Billion in 2030 growing at the CAGR of 10.8% during the forecast period 2023 – 2030.
A biological marker or biomarker is an indicator that measures biological states or conditions. Biomarker testing kits are usually used to detect cancer and also other diseases by looking at genes, proteins, or other substances which can provide information about the disease in different parts of the body and keep track of it during the treatment or after it to help doctors keep track of the disease. Biomarker testing paired with a specific treatment may be called a companion diagnostic test. Biomarker testing is an important part of precision medicine that is an approach to medical care.
Wide prevalence and an increasing number of tumors and cancer in patients across the world, breakthroughs in scientific and technological advancement, and patients opting for non-invasive, efficient, reliable, and cost-effective methods of testing kits have been important factors for the growth of tumor biomarker test kits. Various pharmaceutical companies have been investing in the Research and Development of testing kits which will further be estimated to increase the market of tumor biomarker test kits market during the forecast period.
Global Tumor Biomarker Test Kits Market Drivers:
Growing cases of tumors and a method for their treatment across the world have been fueling the growth of Global Tumor Biomarker Test Kits.
The cases related to tumors and cancers are at an all-time high due to smoking, rising pollution, and poor dietary habits, and are one of the highest reasons for death around the world. Around one-third of the death around due to cancer are related to tobacco usage, alcohol consumption, high body mass index, and lack of physical activity. This is expected to increase the market for Tumor Biomarker Test Kits as people want to detect tumor-related diseases at a very early stage and get treated quickly to decrease the chances of severity.
Increasing cases investments by private companies are also contributing to the growth of the Global Tumor Biomarker Test Kits.
Many Pharmaceutical companies have been investing a lot of capital in research and development of biomarkers test kits to keep advancing and improving the quality of the testing kits as consumers across the world have been opting for a more efficient and cost-effective method of diagnostic and treatment. Various government across the world have been lowering tax rates and subsidies to keep the prices of test kits especially as important as tumor test kits lower so that it is available to a wider range of people. Factors like this are expected to grow the market of Global Tumor Biomarker Test Kits significantly during the forecast period.
Global Tumor Biomarker Test Kits Market Challenges:
Even with the increasing cases of tumors and cancer around the world, breakthroughs in scientific treatments, and the advancement in biomarker testing kits, there has been a lack of reimbursement policies for biomarker tests in developed countries, the high cost of diagnosis and treatment for disease related to tumors in the developing countries is limiting the market as patients are increasingly preferring low-cost detection tests and the number of patients undergoing biomarker treatment is decreasing which can be the major reason that can hamper the growth of Global Tumor Biomarker Test Kits during the forecast period.
Global Tumor Biomarker Test Kits Market Opportunities:
The rapid expansion of biomarker testing services stems from their potential to enhance the development of new and creative medications and biologic products crucial for addressing the ongoing pandemic. These services encompass a range of disease-specific biomarkers that offer quicker and more precise disease identification. This subsequently trims down the timeline for clinical trials in medical research and expedites the overall drug development procedure. Notably, these testing services find extensive application in advancing cancer treatment solutions by detecting alterations in the genetic composition of cells. The anticipated benefits of biomarker testing kits are expected to support the market’s growth during the forecast period.
COVID-19 Impact on Global Tumor Biomarker Test Kits Market:
The pandemic initially hampered research and developmental activities owing to lockdowns and disruption in supply chain management but it opened a new avenue for the market. As people grew more careful about their health and demanded a more efficient, cost-effective, reliable, and quick method of diagnosis and treatment which fuelled the growth of the Tumor Biomarker Test Kits market. With the lifting of lockdowns and life coming back to normal, the research and investments in this sector have again ramped up with a higher growth rate than before. Hence, the overall effect of the COVID-19 pandemic on the Global Tumor Biomarker Test Kits Market was moderate and the market is estimated to show significant growth in the coming future.
Global Tumor Biomarker Test Kits Market Recent Developments:
-
In June 2022, Nonagen Bioscience revealed that its Oncuria immunoassay for bladder cancer has received a CE marking. This pioneering Oncuria test stands out as a multiplex urine analysis capable of quantitatively identifying 10 specific biomarkers linked to the existence of bladder cancer.
-
In February 2022, the OncoDEEP Solid Tumor Biomarker Test Kit has been launched by OncoDNA. This kit, which incorporates enrichment and library preparation solutions from Twist Bioscience, offers laboratories a comprehensive and dependable option for conducting in-depth next-generation sequencing (NGS) analysis on tumor samples.
-
In March 2023, Prestige Biopharma is currently engaged in developing a diagnostic kit specifically crafted for the detection of Pancreatic Adenocarcinoma Upregulated Factor (PAUF). This exceptional biomarker is highly specific to tumors and is found to be overexpressed in around 80% of pancreatic cancer instances, showing a strong connection with early advancement and the spread of the disease. During the application of Receiver Operating Characteristic (ROC) analysis, a method employed to assess the efficacy of a biomarker, PAUF exhibited a significant sensitivity rate of more than 86% when detecting pancreatic cancer.
TUMOR BIOMARKER TEST KITS MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2022 - 2030 |
|
Base Year |
2022 |
|
Forecast Period |
2023 - 2030 |
|
CAGR |
10.8% |
|
By Tumor Detection, Type, Application, Technology, and Region |
|
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Prestige Biopharma, Bio Agilytix Labs, Euro fins Scientific, SGS SA, OncoDEEP, ICON Plc, IQVIA, Syneos Health, Nonagen Bioscience, Intertek Group |
Global Tumor Biomarker Test Kits Market Segmentation: By Tumor Detection
-
Lung
-
Breast
-
Ovarian
-
Liver
-
Stomach
-
Prostate
-
Others
Cancer and tumor in the lungs is the second leading reason for death in the world accounting for more than 13% of all new cases diagnosed in 2022. This has been caused due to increasing smoking of tobacco, exposure to asbestos, certain metals, organic compound, radiation, air pollution, and various other reasons. Diagnostic test kits for lungs have dominated for this segment and are estimated to rise resulting in market expansion in the coming years. Biomarker test kits for breasts have also shown considerable growth for the year 2022 and are estimated to increase during the forecast period.
Global Tumor Biomarker Test Kits Market Segmentation: By Type
-
Protein
-
Genetic
-
Others
The global market was dominated by the Protein segment in 2022 and the segment is poised to maintain its position in the global biomarker market during the forecast period. The research in tumor treatments has been using protein biomarkers which have led to an increase in the market of this segment. Protein biomarkers are one of the most commonly used and accurate measuring biomarkers in the market that can be used to detect various kinds of tumors.
Global Tumor Biomarker Test Kits Market Segmentation: By Application
-
Diagnostic Laboratories
-
Research Institutes
-
Pharmaceutical Companies
The pharmaceutical company’s segment dominated the market and held the highest share of around 45% in 2022. There has also been a rising interest among pharmaceutical companies to develop targeted therapies for tumors Research institutes and diagnostic laboratories have also been significantly contributing to the growth of the market with increased funding of clinical research. Pharmaceutical companies are estimated to maintain their position as the most dominant segment in the market while research institutes and diagnostic laboratories are estimated to show considerable growth in the market during the forecast period.
Global Tumor Biomarker Test Kits Market Segmentation: By Technology
-
Immunoassay
-
Imaging
-
Omics
-
Others
The Omics segment has dominated the market share in 2022. Due to its high efficiency in the early diagnosis of tumors, patients prefer to use the Omics technology. Further research and development and investment in this technology are estimated to grow the market of this biomarker technology. There has also been a growing demand for Immunoassay biomarker technology which is estimated to rise significantly in the coming future as a growing number of clinical trials and drug discovery activities are using immunoassay biomarker technology.
Global Tumor Biomarker Test Kits Market Segmentation: By Region
-
North America
-
Europe
-
Asia-Pacific
-
South America
-
Middle East & Africa
North America held the highest market share of more than 45% in 2022. The market in this region is bolstered by several factors, including the presence of prominent market players offering biomarker testing services, an uptick in the prevalence of diseases prompting a surge in laboratory diagnostic testing, the existence of sophisticated healthcare infrastructure, and a rising level of research and development investment from public organizations within the region. Asia-Pacific region is also estimated to witness one of the fastest growth during the forecast period.
Global Tumor Biomarker Test Kits Market Key Players:
-
Prestige Biopharma
-
Bio Agilytix Labs
-
Euro fins Scientific
-
SGS SA
-
OncoDEEP
-
ICON Plc
-
IQVIA
-
Syneos Health
-
Nonagen Bioscience
-
Intertek Group
Chapter 1. Tumor Biomarker Test Kits Market – Scope & Methodology
1.1 Market Segmentation
1.2 Assumptions
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Tumor Biomarker Test Kits Market – Executive Summary
2.1 Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.3 COVID-19 Impact Analysis
2.3.1 Impact during 2023 – 2030
2.3.2 Impact on Supply – Demand
Chapter 3. Tumor Biomarker Test Kits Market – Competition Scenario
3.1 Market Share Analysis
3.2 Product Benchmarking
3.3 Competitive Strategy & Development Scenario
3.4 Competitive Pricing Analysis
3.5 Supplier - Distributor Analysis
Chapter 4. Tumor Biomarker Test Kits Market - Entry Scenario
4.1 Case Studies – Start-up/Thriving Companies
4.2 Regulatory Scenario - By Region
4.3 Customer Analysis
4.4 Porter's Five Force Model
4.4.1 Bargaining Power of Suppliers
4.4.2 Bargaining Powers of Customers
4.4.3 Threat of New Entrants
4.4.4 .Rivalry among Existing Players
4.4.5 Threat of Substitutes
Chapter 5. Tumor Biomarker Test Kits Market - Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Tumor Biomarker Test Kits Market - By Tumor Detection
6.1 Lung
6.2 Breast
6.3 Ovarian
6.4 Liver
6.5 Stomach
6.6 Prostate
6.7 Others
Chapter 7. Tumor Biomarker Test Kits Market - By Type
7.1 Protein
7.2 Genetic
7.3 Others
Chapter 8. Tumor Biomarker Test Kits Market - By Application
8.1 Diagnostic Laboratories
8.2 Research Institutes
8.3 Pharmaceutical Companies
Chapter 9. Tumor Biomarker Test Kits Market - By Technology
9.1 Immunoassay
9.2 Imaging
9.3 Omics
9.4 Others
Chapter 10. Tumor Biomarker Test Kits Market – By Region
10.1 North America
10.2 Europe
10.3 Asia-Pacific
10.4 Latin America
10.5 The Middle East
10.6 Africa
Chapter 11. Tumor Biomarker Test Kits Market – Key Players
11.1 Prestige Biopharma
11.2 Bio Agilytix Labs
11.3 Euro fins Scientific
11.4 SGS SA
11.5 OncoDEEP
11.6 ICON Plc
11.7 IQVIA
11.8 Syneos Health
11.9 Nonagen Bioscience
11.10 Intertek Group
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The global Tumor Biomarker Test Kits Market is estimated to be worth USD 13.1 Billion in 2022 and is projected to reach the value of USD 29.8 Billion in 2030 growing at the CAGR of 10.8% during the forecast period 2023 – 2030
Rise in several tumors across the world and increasing research and developmental activities coupled with investment by companies.
Based on Type, the Global Tumor Biomarker Test Kits are segmented into Protein, Genetic, and Others.
The United States is the most dominating country in the region of North America for the Global Tumor Biomarker Test Kits Market.
Prestige Biopharma, Bio Agilytix Labs, Euro fins Scientific, SGS SA, OncoDEEP, ICON Plc, IQVIA, Syneos Health, Nonagen Bioscience, Intertek Group