Sterility Test Market Size (2024 – 2030)
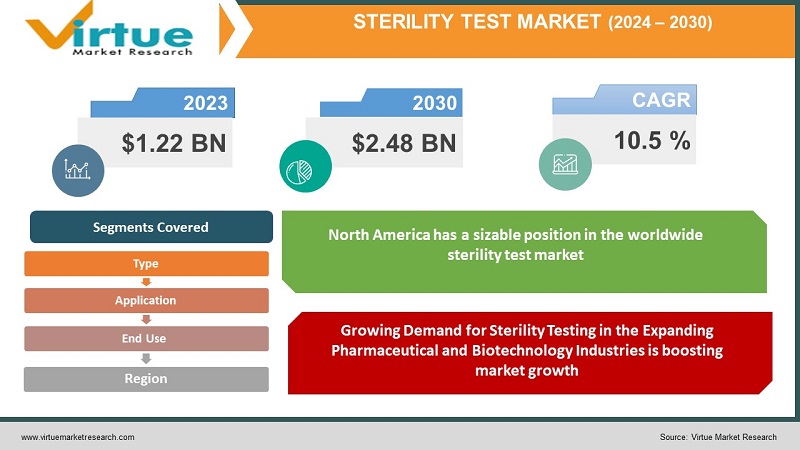
The Global Sterility Test Market was valued at USD 1.22 Billion and is projected to reach a market size of USD 2.45 Billion by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 10.5%.
The market for testing the sterility (lack of live microorganisms) of pharmaceuticals, medical equipment, and other healthcare-related materials is referred to as the worldwide sterility test market. To guarantee the security and caliber of items that come into touch with patients, sterility testing is an essential procedure in the healthcare sector. Sterility tests are done to ensure that a substance is devoid of living germs that might infect users or produce other negative side effects. Pharmaceuticals, including injectable medications, vaccines, and biologics, as well as medical equipment, including implants, surgical tools, and packaging materials, are frequently the subjects of this testing. The market for sterility tests encompasses a wide range of goods, including testing kits, reagents, and tools, as well as services offered by contract testing companies. Hospitals, clinics, research facilities, academic institutions, makers of medical devices, pharmaceutical and biotechnology firms, and other businesses are served by these goods and services. The market is impacted by several variables, including rising regulatory criteria for product safety, escalating demand for drugs and medical equipment, improvements in testing technology, and the desire to adhere to international standards. To fulfill the demands of the healthcare sector, businesses in the sterility test market work to provide precise and dependable testing techniques, provide cutting-edge solutions, and guarantee regulatory compliance. The expansion of the pharmaceutical and biotechnology industries, the growing emphasis on patient safety, and the rising need for quality assurance in healthcare goods are some of the reasons driving the anticipated growth of the worldwide sterility test market in the upcoming years. Based on variables including product type, application, end-user, and geography, market research studies assess the size, trends, and development potential of the sterility test market.
Global Sterility Test Market Drivers:
Growing Demand for Sterility Testing in the Expanding Pharmaceutical and Biotechnology Industries is boosting market growth:
For patient safety, it is essential to ensure the sterility of pharmaceutical items, medical equipment, and healthcare supplies. The demand for strong sterility testing procedures and accurate testing techniques is driven by the increasing awareness of and emphasis on patient safety. Sterility testing is subject to stringent regulations and requirements set by regulatory bodies to guarantee the security and effectiveness of drugs and medical equipment. The implementation of sterility testing and the continuing development of testing procedures are both heavily influenced by compliance with these standards. Growth in the population, aging populations, and the incidence of chronic illnesses are some of the drivers driving the pharmaceutical and biotechnology sectors. To preserve the quality and safety of the products, this expansion feeds the demand for sterility testing.
Technological Advancements Drive the Adoption of Efficient Sterility Testing Methods in Growing Pharmaceutical and Biotechnology Sectors:
Many manufacturers of pharmaceuticals and medical devices contract out their sterility testing to specialist testing businesses. Cost-effectiveness, accessibility to specialized knowledge, and the requirement to concentrate on core capabilities are some of the elements driving this trend. The market for sterility tests is expanding due to the need for contract testing services. Technology developments have resulted in the creation of sterility testing techniques that are more effective and precise. The commercial use of automated microbial detection systems, molecular-based approaches, and quick testing methodologies has increased the efficiency and accuracy of sterility testing. To assure the safety of the final product, stringent sterility testing is necessary in light of the rising demand for biological medicines including monoclonal antibodies and recombinant proteins as well as cell treatments and gene therapies. The market for sterility tests is expanding as a result of the rise of these industries.
Global Sterility Test Market Challenges:
The market for sterility tests is heavily regulated, with stringent rules and specifications imposed by regulatory bodies like the FDA and EMA. It may be difficult and expensive for businesses to comply with these requirements, necessitating investments in advanced testing techniques, qualified employees, and strong quality systems. Numerous sample categories, including pharmaceuticals, medical equipment, and biological materials, are used in sterility testing. These samples frequently have complicated matrices, which might obstruct testing or make it difficult to find microorganisms. It might be difficult to create adequate testing procedures and get over matrix interferences. The prolonged incubation times necessary for microbial growth might cause expenses to rise and product release dates to be pushed back. Traditional sterility testing techniques rely on the development of microorganisms in culture media, which may not be able to identify microorganisms that are not culturable or that are viable but not culturable. Although they may go undetected, these bacteria might nonetheless be a danger to the product's safety. It is still difficult to create trustworthy techniques for detecting non-culturable bacteria.
COVID-19 Impact on Global Sterility Test Market:
Sterile test demand increased dramatically as a result of the pressing need for COVID-19 diagnostics, therapies, and vaccinations. To assure the safety and effectiveness of COVID-19-related goods, pharmaceutical firms, and contract testing organizations increased their sterility testing capabilities. Rapid sterility testing of vaccine candidates was necessary due to the rush around the world to create COVID-19 vaccines. The testing procedure has to be accelerated by the manufacturers while still strictly adhering to regulatory regulations. The supply chain for sterility testing supplies, reagents, and tools was impacted by the worldwide lockdowns, travel restrictions, and transportation difficulties. This caused delays and difficulties in locating key parts, which had an impact on the entire sterility testing procedure. To hasten the approval process for COVID-19-related medicines, regulatory bodies including the FDA and EMA implemented procedures. To enable prompt product availability, these initiatives included streamlining the sterility testing standards and speeding up the approval process. Regulations inspections and audits were carried out remotely due to travel limitations and safety concerns. As a result, businesses had to adjust to virtual inspections and use remote means to show that they complied with regulatory standards, which influenced the sterility testing procedure.
Global Sterility Test Market Recent Developments:
-
In June 2022, Merck announced the addition of additional sterility testing solutions to its BioReliance® portfolio. Pharmaceutical firms may assure the security of their goods by using these devices, which are intended to deliver faster and more precise sterility testing results.
-
In January 2022, Thermo Fisher Scientific unveiled its new Sterilin® line of products, which features an extensive selection of disposable sterility testing plates, closures, and containers. Sterilin® products are designed to improve the effectiveness and dependability of sterility testing procedures.
-
In April 2021, The Sterility Testing Center of Excellence in Lincolnshire, Illinois, expanded, according to SGS. To improve SGS's sterility testing capabilities and serve its customers in the pharmaceutical and biotechnology industries, the expansion involved the installation of new laboratory space and cutting-edge technologies.
STERILITY TEST MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
10.5% |
|
Segments Covered |
By Type, Application, End-User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Merck KGaA, Charles River Laboratories International, Inc., Thermo Fisher Scientific, Inc., SGS SA, bioMérieux SA, Lonza Group Ltd., WuXi AppTec Co., Ltd., Nelson Laboratories, LLC (a part of Sterigenics International LLC), Pace Analytical Services, LLC, Toxikon Corporation |
Global Sterility Test Market Segmentation: By Type
-
Kits and Reagents
-
Instruments
-
Services
These kits frequently include growth promotion assays, filtration membranes, culture medium, and other elements required for performing sterility testing. Because kits and reagents are necessary equipment for conducting sterility testing in a variety of sectors, a sizeable market share is anticipated for this sector. The systems and tools for sterility testing are included in this part. It comprises microbial detection systems, autoclaves, incubators, filtration systems, and other laboratory tools necessary for performing sterility tests. The instruments market is anticipated to expand steadily as a result of increasing technological developments that increase the speed, precision, and automation of sterility testing procedures. CTOs offer sterility testing services to pharmaceutical firms, producers of medical equipment, and other parties involved in healthcare who might not have access to internal testing resources. As more businesses choose to outsource sterility testing to CTOs to concentrate on their core competencies and cut operating expenses, the services industry is expected to expand.
Global Sterility Test Market Segmentation: By Application
-
Pharmaceutical Products
-
Biotechnology Products
-
Medical Devices
-
Healthcare Facilities
Screening for sterility of parenteral medications, such as vaccinations, medicines, and biologics. Eye drops, ointments, creams, and other topical medicinal formulations are sterility tested. Inhalable drug sterility testing, including for nebulizers and inhalers. Pharmaceuticals in powder form, such as antibiotics, antifungals, and painkillers, are subjected to sterility testing. Monoclonal antibody treatments, particularly those used to treat cancer and autoimmune illnesses, undergo sterility testing. Cell-based therapies, such as gene and stem cell treatments, are subjected to sterility testing. Implantable medical equipment, such as orthopedic implants, cardiac devices, and dental implants, are subjected to sterility testing. Scalpels, forceps, and retractor tools are subjected to sterility testing. Catheters, tubes, and other medical instruments used for fluid delivery or drainage undergo sterility testing. Testing for sterility of adhesive bandages, wound dressings, and other wound care supplies. Surgical trays, infusion sets, and wound care devices have all undergone sterility testing before being utilized in hospitals. Sterility testing of medical supplies and equipment used in outpatient settings, such as syringes, blood pressure monitors, and diagnostic tools.
Global Sterility Test Market Segmentation: By End-User
-
Pharmaceutical and Biotechnology Companies
-
Medical Device Manufacturers
-
Hospitals and Clinics
-
Contract Testing Organizations
-
Others
Pharmaceutical producers and biotechnology firms engaged in the creation, manufacture, and distribution of pharmaceutical goods, such as biologics, injectable medications, and vaccines. To make sure that their goods are free from microbial contamination and satisfy regulatory criteria before being released to the market, many businesses require sterility testing. Organizations that produce medical devices such as implants, surgical instruments, catheters, and diagnostic tools. Medical instruments, equipment, and supplies used in healthcare settings are subjected to sterility testing to make sure they are free of microbial contamination and suitable for patient usage. Pharmaceutical, biotechnology, and medical device firms have specialist sterility testing services of contract testing organizations. Companies can outsource their testing needs thanks to these businesses' knowledge, infrastructure, and resources for sterility testing. Sterility testing may also be carried out by academic and research institutes for instructional or research objectives. To support research initiatives, educational initiatives, and quality control procedures, these organizations may have specialist laboratories outfitted for sterility testing.
Global Sterility Test Market Segmentation: By Region
-
North America
-
Europe
-
Asia Pacific
-
Middle East and Africa
-
South America
Due to the existence of big pharmaceutical and biotechnology businesses, stringent regulatory requirements, and advanced healthcare infrastructure, North America has a sizable position in the worldwide sterility test market. Due to its well-established pharmaceutical sector, strong regulatory frameworks, and growing attention to patient safety, Europe is a significant market for sterility testing. The market for sterility tests is increasing quickly in this region as a result of the growing pharmaceutical and biotechnology industries, rising healthcare costs, and a huge patient population. The market for sterility tests is expanding in Latin America as a result of rising healthcare product demand, bettering healthcare infrastructure, and government efforts to raise the standard of pharmaceuticals and medical equipment. The region's sterility test market is impacted by elements like the expanding healthcare sector, increased infrastructure spending, and the adoption of strict regulatory standards.
Global Sterility Test Market Key Players:
-
Merck KGaA
-
Charles River Laboratories International, Inc.
-
Thermo Fisher Scientific, Inc.
-
SGS SA
-
bioMérieux SA
-
Lonza Group Ltd.
-
WuXi AppTec Co., Ltd.
-
Nelson Laboratories, LLC (a part of Sterigenics International LLC)
-
Pace Analytical Services, LLC
-
Toxikon Corporation
Chapter 1. STERILITY TEST MARKET - Scope & Methodology
1.1 Market Segmentation
1.2 Assumptions
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. STERILITY TEST MARKET - Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.3 COVID-19 Impact Analysis
2.3.1 Impact during 2024 – 2030
2.3.2 Impact on Supply – Demand
Chapter 3. STERILITY TEST MARKET - Competition Scenario
3.1 Market Share Analysis
3.2 Product Benchmarking
3.3 Competitive Strategy & Development Scenario
3.4 Competitive Pricing Analysis
3.5 Supplier - Distributor Analysis
Chapter 4. STERILITY TEST MARKET - Entry Scenario
4.1 Case Studies – Start-up/Thriving Companies
4.2 Regulatory Scenario - By Region
4.3 Customer Analysis
4.4 Porter's Five Force Model
4.4.1 Bargaining Power of Suppliers
4.4.2 Bargaining Powers of Customers
4.4.3 Threat of New Entrants
4.4.4 Rivalry among Existing Players
4.4.5 Threat of Substitutes
Chapter 5. STERILITY TEST MARKET - Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. STERILITY TEST MARKET - By Type
6.1 Kits and Reagents
6.2 Instruments
6.3 Services
Chapter 7. STERILITY TEST MARKET - By Application
7.1 Pharmaceutical Products
7.2 Biotechnology Products
7.3 Medical Devices
7.4 Healthcare Facilities
Chapter 8. STERILITY TEST MARKET - By End-User
8.1 Pharmaceutical and Biotechnology Companies
8.2 Medical Device Manufacturers
8.3 Hospitals and Clinics
8.4 Contract Testing Organizations
8.5 Others
Chapter 9. STERILITY TEST MARKET – By Region
9.1 North America
9.2 Europe
9.3 Asia-Pacific
9.4 Latin America
9.5 The Middle East
9.6 Africa
Chapter 10. STERILITY TEST MARKET – Key players
10.1 Merck KGaA
10.2 Charles River Laboratories International, Inc.
10.3 Thermo Fisher Scientific, Inc.
10.4 SGS SA
10.5 bioMérieux SA
10.6 Lonza Group Ltd.
10.7 WuXi AppTec Co., Ltd.
10.8 Nelson Laboratories, LLC (a part of Sterigenics International LLC)
10.9 Pace Analytical Services, LLC
10.10 Toxikon Corporation
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The Global Sterility Test Market was valued at USD 1.22 Billion and is projected to reach a market size of USD 2.45 Billion by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 10.5%.
The Global Sterility Test Market is driven by Growing Demand for Biopharmaceuticals, Growing Demand for Biopharmaceuticals, and Growing Demand for Biopharmaceuticals.
The Segments under the Global Sterility Test Market by the Application are Pharmaceutical Products, Biotechnology Products, Medical Devices, and Healthcare Facilities.
China, Japan, South Korea, Singapore, and India are the most dominating countries in the Asia Pacific region for the Global Sterility Test Market.
Merck KGaA, Charles River Laboratories International, Inc., and Thermo Fisher Scientific, Inc. Company are the three major leading players in the Global Sterility Test Market.



