Chapter 1. SPINAL IMPLANTS MARKET– Scope & Methodology
1.1. Market Segmentation
1.2. Assumptions
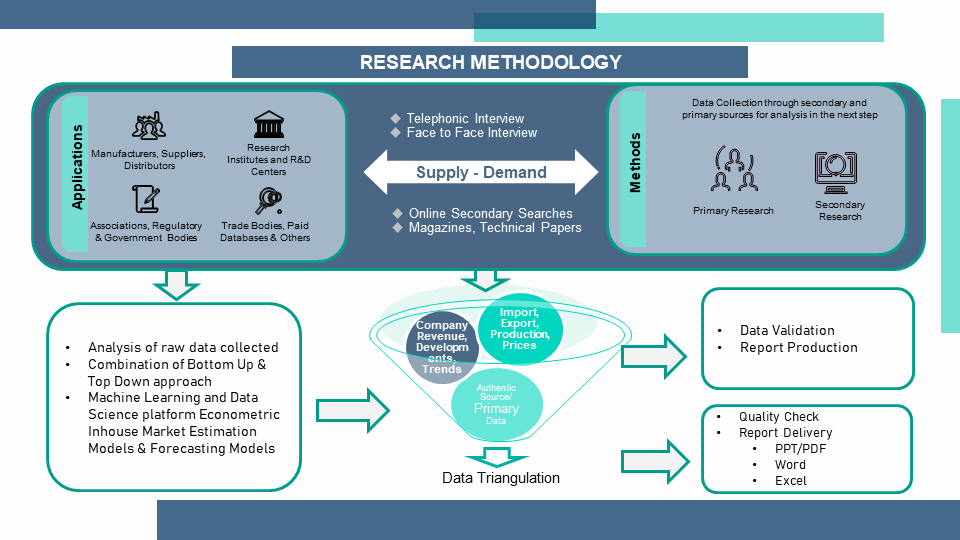
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2. SPINAL IMPLANTS MARKET– Executive Summary
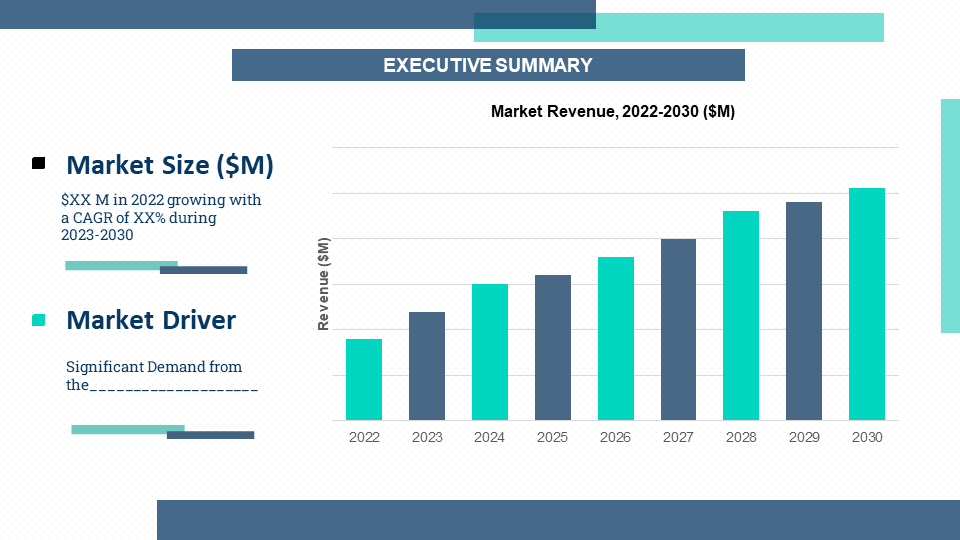
2.1. Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-211 Impact Analysis
2.3.1. Impact during 2023 – 2030
2.3.2. Impact on Supply – Demand
Chapter 3. SPINAL IMPLANTS MARKET– Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4. SPINAL IMPLANTS MARKET- Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatory Scenario - By Region
4.3 Customer Analysis
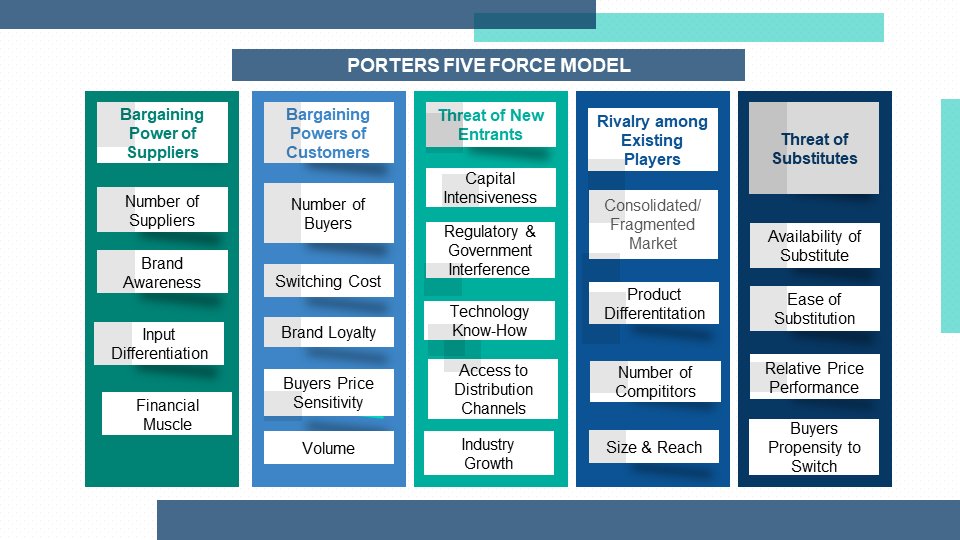
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. SPINAL IMPLANTS MARKET- Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
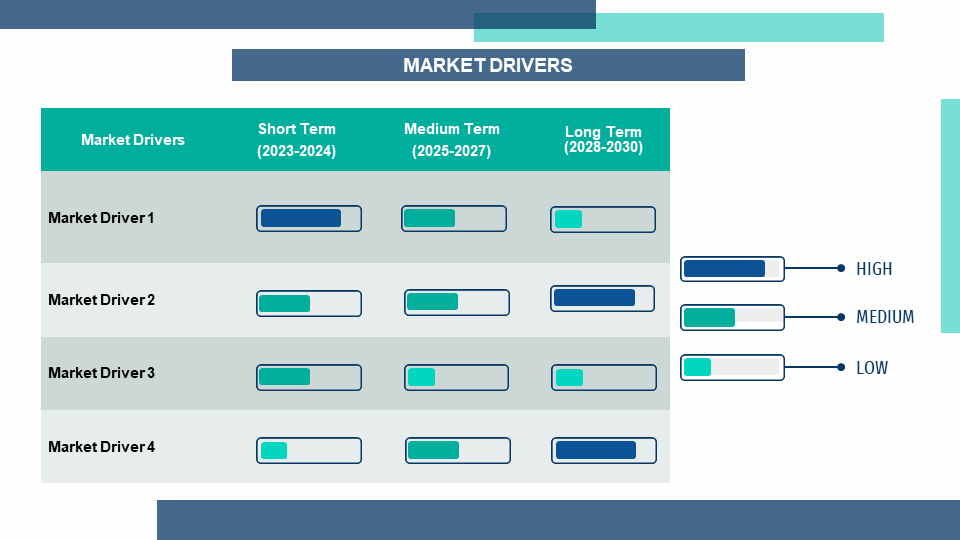
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6. SPINAL IMPLANTS MARKET– By Procedure
6.1. Minimally Invasive Surgery
6.2. Open Surgery
6.3. Others
Chapter 7. SPINAL IMPLANTS MARKET– By Product Type
7.1. Artificial Discs
7.2. Dynamic Stabilization Devices
7.3. Spinal Fusion Implants
Chapter 8. SPINAL IMPLANTS MARKET– By Thoracolumbar Devices
8.1. Anterior lumbar plates
8.2. Cables
8.3. Crosslinks
8.4. Hooks
8.5. Lumar plates
8.6. Pedicle screw
8.7. Rods
8.8. Wires
Chapter 9. SPINAL IMPLANTS MARKET– By Cervical Fixation Devices
9.1. Anterior Cervical Plates
9.2. Bone Interbody Fusion Devices
9.3. Cages
9.4. Clamps and Wires
9.5. Hook Fixation Systems
9.6. Interbody Fusion Devices
9.7. Non-bone Interbody Fusion Devices
9.8. Plates
9.9. Plates and Screws
Chapter 10. SPINAL IMPLANTS MARKET– By Application
10.1. Cervical
10.2. Lumber
10.3. Thoracic
Chapter 11. SPINAL IMPLANTS MARKET– By Material
11.1. Cobalt Chrome
11.2. Polyetheretherketone (PEEK)
11.3. Stainless Steel
11.4. Titanium
Chapter 12. SPINAL IMPLANTS MARKET– By Indication
12.1. Deformity
12.2. Spinal Trauma
Chapter 13. SPINAL IMPLANTS MARKET– By Configuration
13.1. Non-Fusion Devices/Motion Preservation Devices
13.2. Spinal Fusion Devices
Chapter 14. SPINAL IMPLANTS MARKET– By Dynamic Stabilization Devices
14.1. Facet Replacement Products
14.2. Interspinous Process Spacers
14.3. Pedicle Screw-based Systems
Chapter 15. SPINAL IMPLANTS MARKET– By Artificial Discs
15.1. Artificial Cervical Discs
15.2. Artificial Lumbar Discs
Chapter 16. SPINAL IMPLANTS MARKET– By Annulus Repair Devices
16.1. Nuclear Disc Prostheses
16.2. Spinal Bone Stimulators
16.3. Vertebral Compression Fracture (VCF) Treatment Devices
Chapter 17. SPINAL IMPLANTS MARKET– By Non-Invasive Spine Bone Stimulators
17.1. Capacitive Coupling (CC)
17.2. Combined Magnetic field (CMF) Devices
17.3. Pulsed Electromagnetic Field Device
Chapter 18. SPINAL IMPLANTS MARKET– By Invasive Spine Bone Stimulators
18.1. Demineralized Bone Matrix
18.2. Machined Bones Allograft
18.3. Spinal allografts
18.4. Spine Biologics
Chapter 19. SPINAL IMPLANTS MARKET– By Graft Substitutes
19.1. Bone Morphogenetic Proteins
19.2. Cell-based Matrix
19.3. Synthetic Bone Grafts
Chapter 20. SPINAL IMPLANTS MARKET– By Region
20.1. North America
20.2. Europe
20.3. The Asia Pacific
20.4. Latin America
20.5. Middle-East and Africa
Chapter 21. SPINAL IMPLANTS MARKET – Company Profiles – (Overview, Product Portfolio, Financials, Developments)
21.1. Company 1
21.2. Company 2
21.3. Company 3
21.4. Company 4
21.5. Company 5
21.6. Company 6
21.7. Company 7
21.8. Company 8
21.9. Company 9
21.10. Company 10
Download Sample
Choose License Type
2500
4250
5250
6900



