Serology Tests For Syphilis Tests Market Size (2024-2030)
The Serology Tests For Syphilis Tests Market was valued at USD 0.54 billion in 2023 and is projected to reach a market size of USD 0.79 billion by the end of 2030. Over the cast period of 2024 – 2030, the figure for requests is projected to grow at a CAGR of 5.6%.
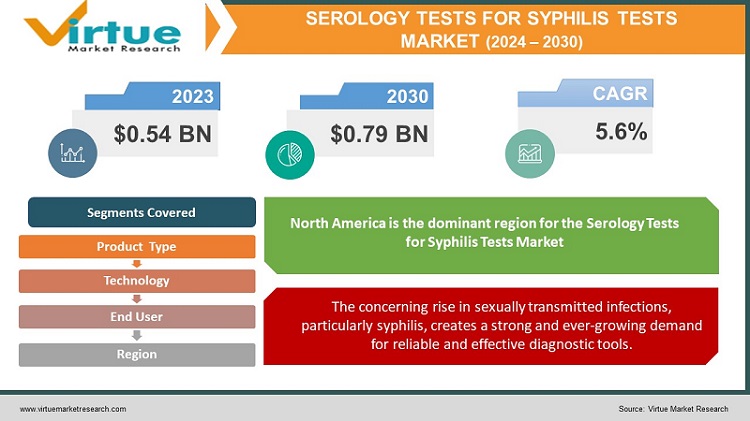
The serology tests for syphilis market is fueled by several factors. The rising number of sexually transmitted diseases, particularly syphilis, creates a strong demand for reliable diagnostic tools. Public health campaigns and educational initiatives are effectively raising awareness about the importance of getting tested, especially among high-risk groups. Additionally, the development of faster and more accurate tests, like rapid syphilis tests, improves access to testing and allows for earlier diagnosis and treatment.
Key Market Insights:
The serology tests for syphilis market is thriving due to a confluence of critical factors. The most concerning factor is the rising prevalence of sexually transmitted infections, particularly syphilis. This has led to a surge in demand for reliable diagnostic tools, and this demand is expected to keep climbing. Public health initiatives are effectively addressing this need by raising awareness about the importance of getting tested, especially among high-risk groups. Studies suggest this awareness can significantly increase the pool of potential test recipients.
Secondly, the market is being revolutionized by advancements in diagnostic technology. The development of faster and more accurate tests, like rapid syphilis tests, is a breakthrough. These tests can provide results in minutes, compared to traditional methods that may take longer. This translates to easier access to testing and earlier diagnosis. Similar segments of the syphilis testing market have shown a potential growth rate of over 5%, indicating a promising future for serology tests. Earlier diagnosis allows for prompt treatment, significantly improving patient outcomes and preventing serious complications associated with syphilis.
Finally, leading players in the market are actively involved in developing even better diagnostic solutions. This continuous innovation bodes well for the future accuracy and efficiency of serology tests for syphilis. These advancements ensure a future where syphilis detection is faster, more precise, and readily available, contributing to a healthier future.
The Serology Tests For Syphilis Tests Market Drivers:
The concerning rise in sexually transmitted infections, particularly syphilis, creates a strong and ever-growing demand for reliable and effective diagnostic tools.
The most concerning factor driving the serology tests for syphilis market is the alarming rise in sexually transmitted infections (STIs), particularly syphilis. This surge in STIs has created a strong and ever-growing demand for reliable and effective diagnostic tools. As the number of syphilis cases continues to climb, the need for accurate and accessible testing becomes increasingly crucial for controlling the spread of the disease and ensuring timely treatment.
Public health initiatives and educational programs are playing a pivotal role in raising public awareness about the importance of syphilis testing, especially among high-risk groups.
Public health initiatives and educational programs are playing a pivotal role in raising public awareness about the importance of syphilis testing, especially among high-risk groups. These comprehensive campaigns are effectively reaching a wider audience, dispelling myths and misconceptions surrounding STIs, and encouraging individuals to get tested. This heightened awareness translates to a larger pool of individuals seeking testing, which ultimately fuels market growth for serology tests.
Advancements in technology are revolutionizing the way syphilis is detected through faster, more accurate, and potentially point-of-care serology tests.
Advancements in technology are revolutionizing the way syphilis is detected. The development of faster, more accurate, and potentially point-of-care serology tests is a major breakthrough in the field of STI diagnostics. These innovative tests, such as rapid syphilis tests, can provide results in a matter of minutes, compared to traditional methods that may take longer. This translates to significant benefits for both patients and healthcare providers. Easier and faster access to testing, particularly through point-of-care options, can significantly increase testing rates and facilitate earlier diagnoses.
Early detection of syphilis plays a crucial role in preventing the development of serious complications, with innovative serology tests enabling prompt treatment interventions.
Early detection of syphilis plays a crucial role in preventing the development of serious complications associated with the disease. Innovative serology tests that enable earlier diagnosis to allow for prompt treatment interventions, significantly improving patient outcomes. By detecting syphilis at an earlier stage, healthcare providers can prevent the infection from progressing and causing long-term damage to the body. This focus on early detection through accurate and accessible serology tests is a key driver of market growth.
The Serology Tests For Syphilis Tests Market Restraints and Challenges:
The serology tests for syphilis market, while promising, faces some hurdles. One challenge is limited test specificity. Though widely used, some tests might yield false positives, causing unnecessary anxiety and extra testing for patients. Additionally, even with advancements, some tests may not detect syphilis in the very early stages, delaying diagnosis and treatment.
Another hurdle is the social stigma surrounding STIs. This discourages people from getting tested, even if they are aware of its importance, limiting the market reach. Furthermore, the cost of these tests can be a barrier, particularly in areas with limited resources. This can hinder wider access and slow down-market growth.
Finally, traditional serology tests often rely on trained healthcare professionals for administration and interpretation. This can limit access to testing in regions with healthcare worker shortages. Despite these challenges, the market is expected to grow due to the rising prevalence of STIs, increasing public awareness, and continuous advancements in diagnostic technology.
The Serology Tests For Syphilis Tests Market Opportunities:
The serology tests for syphilis market presents exciting opportunities for growth. Firstly, there's a chance to develop highly specific tests, reducing false positives and unnecessary procedures for patients. Secondly, advancements in technology hold promise for early detection tests, allowing for prompt treatment and minimizing complications.
Public health initiatives can also play a role by tackling the stigma surrounding STIs. Open communication and education can encourage individuals to get tested without fear, expanding the market.
Furthermore, developing cost-effective tests is crucial for wider access, especially in resource-limited areas. This could involve exploring alternative testing methods or government subsidies. Additionally, the rise of point-of-care tests presents a significant opportunity. These rapid tests can be administered in various settings, increasing access for remote areas and high-risk populations.
Finally, continuous advancements in technology hold promise for the future. Faster, more accurate, and potentially self-administered tests could be developed, revolutionizing syphilis detection and management. By capitalizing on these opportunities, the serology tests for syphilis market can significantly contribute to controlling the spread of the disease and ensuring timely treatment.
SEROLOGY TESTS FOR SYPHILIS TESTS MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
5.6% |
|
Segments Covered |
By Product Type, Technology, end user, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Abbott Laboratories, F. Hoffmann-La Roche Ltd, Siemens Healthineers AG, DiaSorin S.p.A., Becton Dickinson and Company, Danaher (Beckman Coulter, Inc.), Bio-Rad Laboratories, Inc. |
The Serology Tests For Syphilis Tests Market Segmentation:
The Serology Tests For Syphilis Tests Market Segmentation: By Product Type:
- Non-Treponemal Tests
- Treponemal Tests
The dominant segment in the serology tests for syphilis market by product type is currently Non-Treponemal Tests. These tests are widely used for initial screening due to their faster turnaround time and lower cost. However, the fastest-growing segment is expected to be Rapid Tests. Their point-of-care functionality and ability to provide results in minutes are increasing access to testing, particularly in resource-limited settings.
The Serology Tests For Syphilis Tests Market Segmentation: By Technology:
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Chemiluminescence Immunoassay (CLIA)
- Rapid Tests
The Enzyme-Linked Immunosorbent Assay (ELISA) segment is currently the most dominant technology in the serology tests for syphilis market due to its widespread adoption and cost-effectiveness. However, the fastest-growing segment is expected to be Rapid Tests. These point-of-care tests offer quicker results and increased access to testing in various settings, making them ideal for resource-limited areas or high-risk populations.
The Serology Tests For Syphilis Tests Market Segmentation: By End User:
- Hospitals & Clinics
- Diagnostic Laboratories
- Blood Banks
- Public Health Facilities
Hospitals & Clinics are the dominant segment in the serology tests for syphilis market due to their established role in diagnosing and treating various diseases, including syphilis. Public Health Facilities are expected to be the fastest-growing segment due to a growing focus on disease surveillance and control efforts to curb the spread of STIs like syphilis.
The Serology Tests For Syphilis Tests Market Segmentation: Regional Analysis:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East and Africa
North America: This region holds the dominant position in the serology tests for syphilis market due to several factors. High public awareness about STIs, established healthcare infrastructure with well-equipped hospitals and clinics, and strong government support for public health initiatives all contribute to market growth. Additionally, the presence of leading diagnostic companies’ further fuels research and development in this region.
Europe: European nations boast developed healthcare systems with a strong focus on diagnostic testing. Government initiatives promoting STI screening and readily available healthcare facilities contribute to market expansion. However, regional variations exist, with some countries facing challenges in public awareness and access to testing in remote areas.
Asia-Pacific: This region presents significant growth potential due to a combination of factors. The rising prevalence of STIs, particularly syphilis, necessitates increased testing efforts. Additionally, growing healthcare spending and expanding awareness campaigns are driving market expansion. However, challenges remain, including limited healthcare infrastructure in some areas and a social stigma surrounding STIs that can discourage testing.
Latin America: The Latin American market offers promising opportunities for growth. Similar to the Asia-Pacific region, an increasing number of syphilis cases highlights the need for wider testing initiatives. Growing awareness about STIs and improving healthcare infrastructure in many countries are positive signs. However, limited resources and disparities in healthcare access across the region require further development.
Middle East and Africa: This region faces significant challenges due to limited resources, weak healthcare infrastructure in some areas, and a strong social stigma surrounding STIs. Despite these hurdles, the high prevalence of syphilis underscores the need for increased testing efforts. Government initiatives, along with international aid programs, could play a crucial role in promoting public awareness, improving healthcare access, and fostering market development for serology tests in this region.
COVID-19 Impact Analysis on the Serology Tests For Syphilis Tests Market:
The COVID-19 pandemic has had a complex impact on the serology tests for syphilis market. On the one hand, the pandemic caused disruptions. Diverted healthcare resources towards COVID-19 diagnosis and treatment led to temporary declines in routine STI testing, including syphilis. Lockdowns and social distancing measures might have also discouraged people from visiting clinics for checkups and STI testing. Additionally, global supply chain disruptions could have caused temporary shortages of test kits and reagents.
However, there are also positive aspects to consider. The heightened public awareness about infectious diseases during the pandemic could translate to a long-term increase in awareness about STIs, potentially encouraging more people to get tested for syphilis. The rise of telehealth consultations could offer an alternative pathway for individuals to seek guidance and get referrals for testing. Finally, the pandemic's emphasis on public health measures could lead to increased government funding for STI prevention and control programs, potentially improving access to syphilis testing services.
The overall impact of COVID-19 on the market is likely a mixed bag. While there were short-term disruptions, it could also lead to a long-term increase in awareness and demand for testing. The long-term effects will depend on factors like the effectiveness of public health initiatives, the development of new diagnostic tools, and the overall healthcare landscape in the post-pandemic era.
Latest Trends/ Developments:
The serology tests for syphilis market is experiencing exciting advancements. A key trend is the development of rapid point-of-care (POC) tests. These tests deliver results in minutes, increasing access to testing in various settings beyond traditional clinics. This caters to individuals hesitant to visit healthcare facilities or those in remote areas. Additionally, research is exploring multiplex tests that can detect syphilis alongside other STIs in one go, improving efficiency and providing a more comprehensive picture of sexual health.
Furthermore, the market is witnessing the integration of serology tests with digital platforms. This could involve linking test results to electronic health records and using digital tools for appointments, results delivery, and educational resources.
Finally, there's a growing focus on automation and standardization in the market. Automated testing systems are being developed to improve lab efficiency and reduce errors. Efforts are also underway to standardize testing protocols across regions for consistent and reliable results. The potential of Artificial Intelligence (AI) is also being explored to analyze test results and identify syphilis infection patterns, leading to earlier and more accurate diagnoses. These advancements hold promise for improved access to testing, increased accuracy and efficiency, and ultimately, better management and control of syphilis.
Key Players:
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd
- Siemens Healthineers AG
- DiaSorin S.p.A.
- Becton Dickinson and Company
- Danaher (Beckman Coulter, Inc.)
- Bio-Rad Laboratories, Inc.
Chapter 1. GLOBAL SEROLOGY TESTS FOR SYPHILIS TESTS MARKET– SCOPE & METHODOLOGY
1.1. Market Segmentation
1.2. Scope, Assumptions & Limitations
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2. GLOBAL SEROLOGY TESTS FOR SYPHILIS TESTS MARKET – EXECUTIVE SUMMARY
2.1. Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.2.1. Demand Side
2.2.2. Supply Side
2.3. Attractive Investment Propositions
2.4. COVID-19 Impact Analysis
Chapter 3. GLOBAL SEROLOGY TESTS FOR SYPHILIS TESTS MARKET– COMPETITION SCENARIO
3.1. Market Share Analysis & Company Benchmarking
3.2. Competitive Strategy & Development Scenario
3.3. Competitive Pricing Analysis
3.4. Supplier-Distributor Analysis
Chapter 4. GLOBAL SEROLOGY TESTS FOR SYPHILIS TESTS MARKET - ENTRY SCENARIO
4.1. Regulatory Scenario
4.2. Case Studies – Key Start-ups
4.3. Customer Analysis
4.4. PESTLE Analysis
4.5. Porters Five Force Model
4.5.1. Bargaining Power of Suppliers
4.5.2. Bargaining Powers of Customers
4.5.3. Threat of New Entrants
4.5.4. Rivalry among Existing Players
4.5.5. Threat of Substitutes
Chapter 5. GLOBAL SEROLOGY TESTS FOR SYPHILIS TESTS MARKET- LANDSCAPE
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6. GLOBAL SEROLOGY TESTS FOR SYPHILIS TESTS MARKET– BY PRODUCT TYPE
6.1. Introduction/Key Findings
6.2. Non-Treponemal Tests
6.3. Treponemal Tests
6.4. Y-O-Y Growth trend Analysis By Product Type
6.5. Absolute $ Opportunity Analysis By Product Type , 2024-2030
Chapter 7. GLOBAL SEROLOGY TESTS FOR SYPHILIS TESTS MARKET– BY TECHNOLOGY
7.1. Introduction/Key Findings
7.2. Enzyme-Linked Immunosorbent Assay (ELISA)
7.3. Chemiluminescence Immunoassay (CLIA)
7.4. Rapid Tests
7.5. Y-O-Y Growth trend Analysis By TECHNOLOGY
7.6. Absolute $ Opportunity Analysis By TECHNOLOGY , 2024-2030
Chapter 8. GLOBAL SEROLOGY TESTS FOR SYPHILIS TESTS MARKET– BY End User
8.1. Introduction/Key Findings
8.2. Hospitals & Clinics
8.3. Diagnostic Laboratories
8.4. Blood Banks
8.5. Public Health Facilities
8.6. Y-O-Y Growth trend Analysis End User
8.7. Absolute $ Opportunity Analysis End User , 2024-2030
Chapter 9. GLOBAL SEROLOGY TESTS FOR SYPHILIS TESTS MARKET, BY GEOGRAPHY – MARKET SIZE, FORECAST, TRENDS & INSIGHTS
9.1. North America
9.1.1. By Country
9.1.1.1. U.S.A.
9.1.1.2. Canada
9.1.1.3. Mexico
9.1.2. By TECHNOLOGY
9.1.3. By PRODUCT Type
9.1.4. By End-Use
9.1.5. Countries & Segments - Market Attractiveness Analysis
9.2. Europe
9.2.1. By Country
9.2.1.1. U.K.
9.2.1.2. Germany
9.2.1.3. France
9.2.1.4. Italy
9.2.1.5. Spain
9.2.1.6. Rest of Europe
9.2.2. By TECHNOLOGY
9.2.3. By PRODUCT Type
9.2.4. By End-Use
9.2.5. Countries & Segments - Market Attractiveness Analysis
9.3. Asia Pacific
9.3.1. By Country
9.3.1.1. China
9.3.1.2. Japan
9.3.1.3. South Korea
9.3.1.4. India
9.3.1.5. Australia & New Zealand
9.3.1.6. Rest of Asia-Pacific
9.3.2. By TECHNOLOGY
9.3.3. By PRODUCT Type
9.3.4. By End-Use
9.3.5. Countries & Segments - Market Attractiveness Analysis
9.4. South America
9.4.1. By Country
9.4.1.1. Brazil
9.4.1.2. Argentina
9.4.1.3. Colombia
9.4.1.4. Chile
9.4.1.5. Rest of South America
9.4.2. By TECHNOLOGY
9.4.3. By PRODUCT Type
9.4.4. By End-Use
9.4.5. Countries & Segments - Market Attractiveness Analysis
9.5. Middle East & Africa
9.5.1. By Country
9.5.1.1. United Arab Emirates (UAE)
9.5.1.2. Saudi Arabia
9.5.1.3. Qatar
9.5.1.4. Israel
9.5.1.5. South Africa
9.5.1.6. Nigeria
9.5.1.7. Kenya
9.5.1.8. Egypt
9.5.1.9. Rest of MEA
9.5.2. By TECHNOLOGY
9.5.3. By PRODUCT Type
9.5.4. By End-Use
9.5.5. Countries & Segments - Market Attractiveness Analysis
Chapter 10. GLOBAL SEROLOGY TESTS FOR SYPHILIS TESTS MARKET– COMPANY PROFILES – (OVERVIEW, PRODUCT PORTFOLIO, FINANCIALS, STRATEGIES & DEVELOPMENTS)
10.1 Abbott Laboratories
10.2. F. Hoffmann-La Roche Ltd
10.3. Siemens Healthineers AG
10.4. DiaSorin S.p.A.
10.5. Becton Dickinson and Company
10.6. Danaher (Beckman Coulter, Inc.)
10.7. Bio-Rad Laboratories, Inc.
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The Serology Tests For Syphilis Tests Market was valued at USD 0.54 billion in 2023 and is projected to reach a market size of USD 0.79 billion by the end of 2030. Over the cast period of 2024 – 2030, the figure for requests is projected to grow at a CAGR of 5.6%.
`Escalating Rates of Sexually Transmitted Diseases, Enhanced Public Awareness Efforts, Diagnostic Innovation Revolutionizing Testing, Focus on Early Detection for Improved Outcomes.
Hospitals & Clinics, Diagnostic Laboratories, Blood Banks, Public Health Facilities.
Currently, North America is the dominant region for the Serology Tests for Syphilis Tests Market due to factors like high awareness, established healthcare infrastructure, and strong government support
Abbott Laboratories, F. Hoffmann-La Roche Ltd, Siemens Healthineers AG, DiaSorin S.p.A., Becton Dickinson and Company, Danaher (Beckman Coulter, Inc.), Bio-Rad Laboratories, Inc..



