Sanger-based Diagnostics Testing Market Size (2024 –2030)
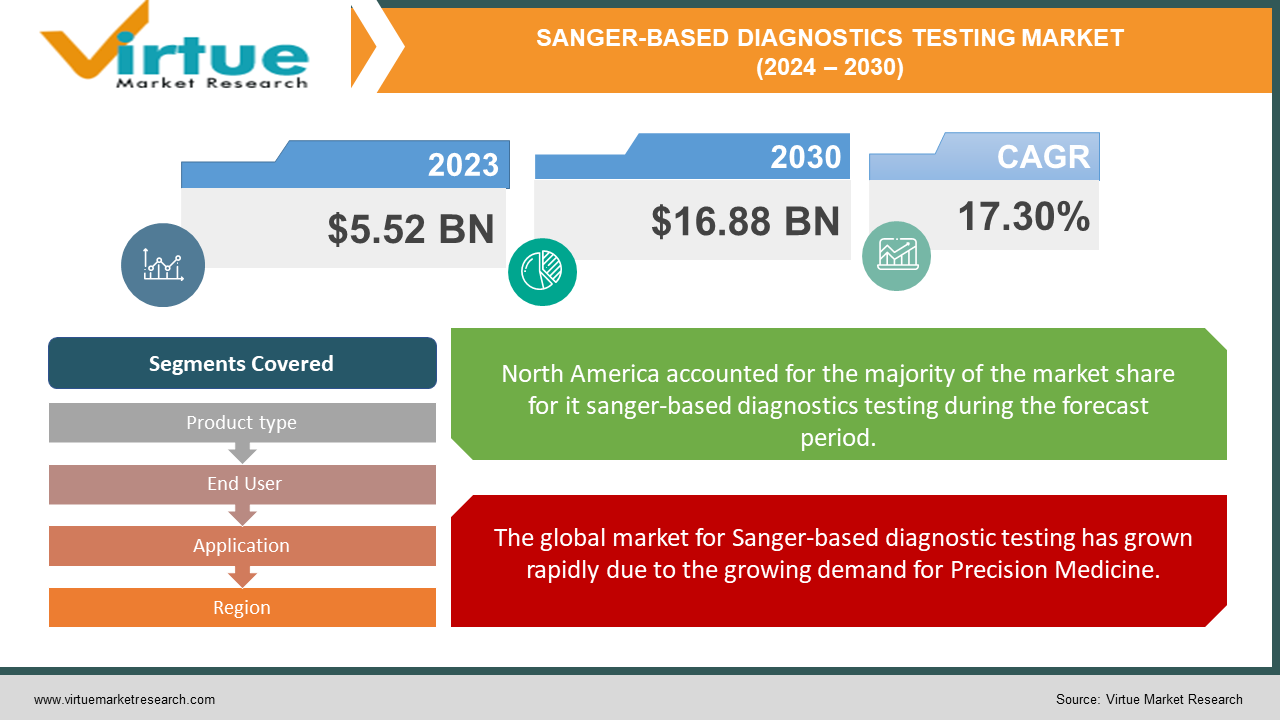
Global Sanger-based Diagnostics Testing Market was valued at USD 5.52 Billion and is projected to reach a market size of USD 16.88 Billion by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 17.30%.
The technique known as "Sanger sequencing," after Frederick Sanger, is used to determine the base-by-base sequence of a DNA strand. It contributed to the sequencing of the human genome and was a breakthrough in the late 1970s. This is how it operates. Consider DNA as an extended sequence of building pieces. Using a unique enzyme known as DNA polymerase, scientists duplicate a particular section of this chain numerous times in Sanger sequencing. They also incorporate altered building blocks known as dideoxynucleotides (ddNTPs), which, upon addition to the chain, halt the replication process due to the absence of a critical component required to complete the construction of the DNA strand. The precise base order in that DNA segment can be determined by scientists by introducing these altered building blocks and tracking the point at which the copying ends. Understanding genetic traits and diseases, conducting medical testing, forensics, and research have all benefited greatly from this technique.
Key Market Insights:
Oncology applications account for approximately 35% of the Sanger-based diagnostics testing market share, driven by the need for accurate genetic profiling in cancer diagnosis and treatment planning.
The clinical laboratories segment constitutes around 60% of the demand for Sanger-based diagnostics testing, reflecting the widespread use of this technology in routine genetic testing and specialized diagnostic services.
In terms of region, North America holds the largest market share of about 45% for Sanger-based diagnostics testing, attributed to the well-established healthcare infrastructure, high adoption of genetic testing, and significant research activities in genomics.
The adoption of automated Sanger sequencing platforms is growing at a rate of approximately 8% annually, driven by the need for higher throughput, improved accuracy, and reduced turnaround times in diagnostic testing.
Global Sanger-based Diagnostics Testing Market Drivers:
The global market for Sanger-based diagnostic testing has grown rapidly due to the growing demand for Precision Medicine.
Precision medicine, also referred to as personalized medicine, is a branch of medicine that customizes care for individual patients based on their genetic composition, lifestyle, and environment. This method seeks to offer safer and more effective medical care than standard approaches that treat all patients equally. Sanger sequencing is essential to precision medicine because it can precisely detect genetic alterations that may result in illness. This enables physicians to tailor treatment regimens according to the genetic profile of each patient. There will likely be a greater need for trustworthy DNA sequencing techniques like Sanger-based testing as precision medicine gains traction. As healthcare providers look for more precise genetic information to provide better, more individualized treatments, this trend is expected to propel significant growth in the global market for Sanger-based diagnostics testing.
The clinical application of Sanger-based diagnostics testing in genetic disorders and oncology research is a significant factor driving the global market.
Sanger sequencing is still a useful technique for determining the presence of genetic disorders and researching cancer. Due to the necessity for precise genetic information and the continuous advancements in disease research, its application in healthcare is predicted to increase globally. Sanger sequencing has a reputation for precisely identifying genetic variants associated with hereditary illnesses. It is essential for making diagnoses, determining the severity of conditions, offering genetic counseling, and initiating preventative care. Sanger sequencing is still used in many genetic studies and is widely trusted in clinical settings due to its accuracy and dependability, which is fueling the demand for Sanger-based testing throughout the world.
Sanger-based Diagnostics Testing Market Challenges and Restraints:
Numerous obstacles could prevent the Sanger-based diagnostic test market from expanding. The advent of increasingly sophisticated sequencing technologies, such as next-generation sequencing (NGS), presents a significant challenge. NGS is widely used in research and diagnostics because it makes sequencing large amounts of DNA faster and less expensive. Sanger sequencing is becoming less necessary due to this trend since NGS can handle larger data sets and detect more intricate genetic alterations. The amount of DNA that Sanger sequencing can process and its ability to identify specific genomic alterations—which are necessary for comprehensive genetic research—are also limited. Labs must train personnel and purchase new equipment in order to transition to NGS, which can be expensive and time-consuming. The market for Sanger-based diagnostics must identify niches in which its approach works well and adapt to the shifting needs of genomic diagnostics in order to remain competitive. Sanger sequencing will have to change as NGS expands in order to remain relevant in the rapidly changing world of genetic testing.
Sanger-based Diagnostics Testing Market Opportunities:
Notwithstanding obstacles, there are growth prospects in the global market for Sanger-based diagnostic testing. One promising strategy is to work in tandem with next-generation sequencing (NGS) technologies, as opposed to competing with them. For certain applications, such as targeted sequencing and NGS result verification, sanger sequencing is still useful. It is particularly good at identifying individual genetic variants, which is critical for precision medicine and supporting significant genomic findings. In order to prosper, Sanger sequencing can concentrate on assisting researchers and medical professionals who require accurate analyses and validation of noteworthy genetic discoveries. Its competitiveness for these specific applications will increase with advancements in automation and data processing, which will also boost efficiency and reduce costs. Accurate genetic testing will become more and more in demand as interest in precision medicine increases. The Sanger-based diagnostics industry can grow and prosper in the changing healthcare environment by carefully aligning itself with emerging sequencing technologies.
SANGER-BASED DIAGNOSTICS TESTING MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
17.30% |
|
Segments Covered |
By Product type, End User, Application, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Thermo Fisher Scientific Inc, Source BioScience, GenScript, Quintara Biosciences, GENEWIZ, LGC Ltd |
Global Sanger-based Diagnostics Testing Market Segmentation: By Product Type
-
Sequencing Instruments
-
Sequencing Consumables
-
Software Solutions
Sequencing instruments presently hold the largest market share and are predicted to continue growing in the upcoming years, based on how products are categorized in the market. Software solutions, on the other hand, are the fastest-growing market and are expected to grow quickly over the course of the projection.
Global Sanger-based Diagnostics Testing Market Segmentation: By End User
-
Hospitals
-
Diagnostic Laboratories
-
Research Institutions
-
Academic Centers
-
Others
Due to their high demand for Sanger-based diagnostic testing, diagnostic laboratories currently generate the most revenue when it comes to who uses these products. The sector with the quickest rate of growth is research institutions, which is predicted to keep growing over the projection period. Their numerous uses in Sanger sequencing and genomic research projects are what are fueling this expansion.
Global Sanger-based Diagnostics Testing Market Segmentation: By Application
-
Disease Diagnostics
-
Biomarkers & Cancer Testing
-
Genetic Disorders
-
Personalized Medicines
-
Forensics
-
Reproductive Health
-
Others
Because of its widespread application in oncology research, the identification of biomarkers, and the increasing prevalence of chronic diseases such as cancer, cancer testing currently holds the largest market share when it comes to the application of Sanger-based diagnostics. The fastest-growing market is disease diagnostics, which is fueled by the accuracy and precision offered by Sanger-based testing techniques.
Global Sanger-based Diagnostics Testing Market Segmentation: By Region
-
North America
-
Europe
-
Asia Pacific
-
Middle East and Africa
-
South America
Due to factors like a growing elderly population and widespread adoption of cutting-edge medical technologies, North America currently dominates the market when it comes to regional segmentation for Sanger-based Diagnostics Testing. In the meanwhile, Asia-Pacific is anticipated to experience substantial growth in the years to come, propelled by rising R&D expenditures and a rise in the regional adoption of Sanger-based diagnostic testing.
COVID-19 Impact on the Global Sanger-based Diagnostics Testing Market:
Both positive and negative effects of the COVID-19 pandemic have been felt by the global Sanger-based diagnostics testing industry. Positively, there has been an increased demand for accurate testing techniques such as Sanger sequencing to detect and monitor SARS-CoV-2 virus variants. Sanger sequencing is now more in demand in the study of infectious diseases as a result. But the pandemic has also interfered with routine medical procedures, which has decreased the need for Sanger sequencing-based non-urgent genetic testing. Furthermore, the production and distribution of Sanger sequencing instruments and materials have been impacted by the pandemic's effects on the economy and supply chains. Delays and higher expenses have resulted from this. There are still chances for the market to bounce back and adjust in spite of these obstacles. Promising avenues for future growth include the specific application of Sanger sequencing in infectious disease research and the heightened focus on disease detection.
Latest Trend/Development:
Recent developments in automation and data processing, which increase testing's effectiveness and affordability, are driving the Sanger-based Diagnostics Testing Market. For thorough genetic analysis, there is also an increasing emphasis on combining Sanger sequencing with other cutting-edge technologies like next-generation sequencing (NGS). Furthermore, Sanger-based testing is in greater demand in precision medicine, as genetically tailored treatments are becoming more prevalent. These patterns point to a dynamic shift in healthcare toward more precise and customized diagnostic solutions.
Key Players:
-
Thermo Fisher Scientific Inc
-
Source BioScience
-
GenScript
-
Quintara Biosciences
-
GENEWIZ
-
LGC Ltd
Market News:
-
Sanger-based diagnostic testing was first made available in San Diego by Source BioScience in August 2022. They bring to the USA decades of experience providing genomic services.
-
In February 2022, Thermo Fisher released the SeqStudio Flex Series genetic analyzer. This new Applied Biosystem improves Sanger-based diagnostics by offering improved connectivity, increased flexibility, ease of use, and remote service options.
Chapter 1. Sanger-based Diagnostics Testing Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Sanger-based Diagnostics Testing Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Sanger-based Diagnostics Testing Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Sanger-based Diagnostics Testing Market - Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Sanger-based Diagnostics Testing Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Sanger-based Diagnostics Testing Market – By Product Type
6.1 Introduction/Key Findings
6.2 Sequencing Instruments
6.3 Sequencing Consumables
6.4 Software Solutions
6.5 Y-O-Y Growth trend Analysis By Product Type
6.6 Absolute $ Opportunity Analysis By Product Type, 2024-2030
Chapter 7. Sanger-based Diagnostics Testing Market – By End-use
7.1 Introduction/Key Findings
7.2 Hospitals
7.3 Diagnostic Laboratories
7.4 Research Institutions
7.5 Academic Centers
7.6 Others
7.7 Y-O-Y Growth trend Analysis By End-use
7.8 Absolute $ Opportunity Analysis By End-use, 2024-2030
Chapter 8. Sanger-based Diagnostics Testing Market – By Application
8.1 Introduction/Key Findings
8.2 Disease Diagnostics
8.3 Biomarkers & Cancer Testing
8.4 Genetic Disorders
8.5 Personalized Medicines
8.6 Forensics
8.7 Reproductive Health
8.8 Others
8.9 Y-O-Y Growth trend Analysis By Application
8.10 Absolute $ Opportunity Analysis By Application, 2024-2030
Chapter 9. Sanger-based Diagnostics Testing Market , By Geography – Market Size, Forecast, Trends & Insights
9.1 North America
9.1.1 By Country
9.1.1.1 U.S.A.
9.1.1.2 Canada
9.1.1.3 Mexico
9.1.2 By Product Type
9.1.3 By End-use
9.1.4 By Application
9.1.5 Countries & Segments - Market Attractiveness Analysis
9.2 Europe
9.2.1 By Country
9.2.1.1 U.K
9.2.1.2 Germany
9.2.1.3 France
9.2.1.4 Italy
9.2.1.5 Spain
9.2.1.6 Rest of Europe
9.2.2 By Product Type
9.2.3 By End-use
9.2.4 By Application
9.2.5 Countries & Segments - Market Attractiveness Analysis
9.3 Asia Pacific
9.3.1 By Country
9.3.1.1 China
9.3.1.2 Japan
9.3.1.3 South Korea
9.3.1.4 India
9.3.1.5 Australia & New Zealand
9.3.1.6 Rest of Asia-Pacific
9.3.2 By Product Type
9.3.3 By End-use
9.3.4 By Application
9.3.5 Countries & Segments - Market Attractiveness Analysis
9.4 South America
9.4.1 By Country
9.4.1.1 Brazil
9.4.1.2 Argentina
9.4.1.3 Colombia
9.4.1.4 Chile
9.4.1.5 Rest of South America
9.4.2 By Product Type
9.4.3 By End-use
9.4.4 By End-use
9.4.5 Countries & Segments - Market Attractiveness Analysis
9.5 Middle East & Africa
9.5.1 By Country
9.5.1.1 United Arab Emirates (UAE)
9.5.1.2 Saudi Arabia
9.5.1.3 Qatar
9.5.1.4 Israel
9.5.1.5 South Africa
9.5.1.6 Nigeria
9.5.1.7 Kenya
9.5.1.8 Egypt
9.5.1.9 Rest of MEA
9.5.2 By Product Type
9.5.3 By End-use
9.5.4 By Application
9.5.5 Countries & Segments - Market Attractiveness Analysis
Chapter 10. Sanger-based Diagnostics Testing Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
10.1 Thermo Fisher Scientific Inc
10.2 Source BioScience
10.3 GenScript
10.4 Quintara Biosciences
10.5 GENEWIZ
10.6 LGC Ltd
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
Global Sanger-based Diagnostics Testing Market was valued at USD 5.52 Billion and is projected to reach a market size of USD 16.88 Billion by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 17.30%
Rising demand for Precision Medicine has led to rapid growth in the global Sanger-based diagnostics testing market.
Sequencing Instruments, Sequencing Consumables and Software Solutions are the segments by product type under the Global Sanger-based Diagnostics Testing Market.
North America dominates the market in the Global Sanger-based Diagnostics Testing Market.
Asia-Pacific is the fastest-growing region in the Global Sanger-based Diagnostics Testing Market.