Rare Genetic Diseases Testing Kit Market Size (2023-2030)
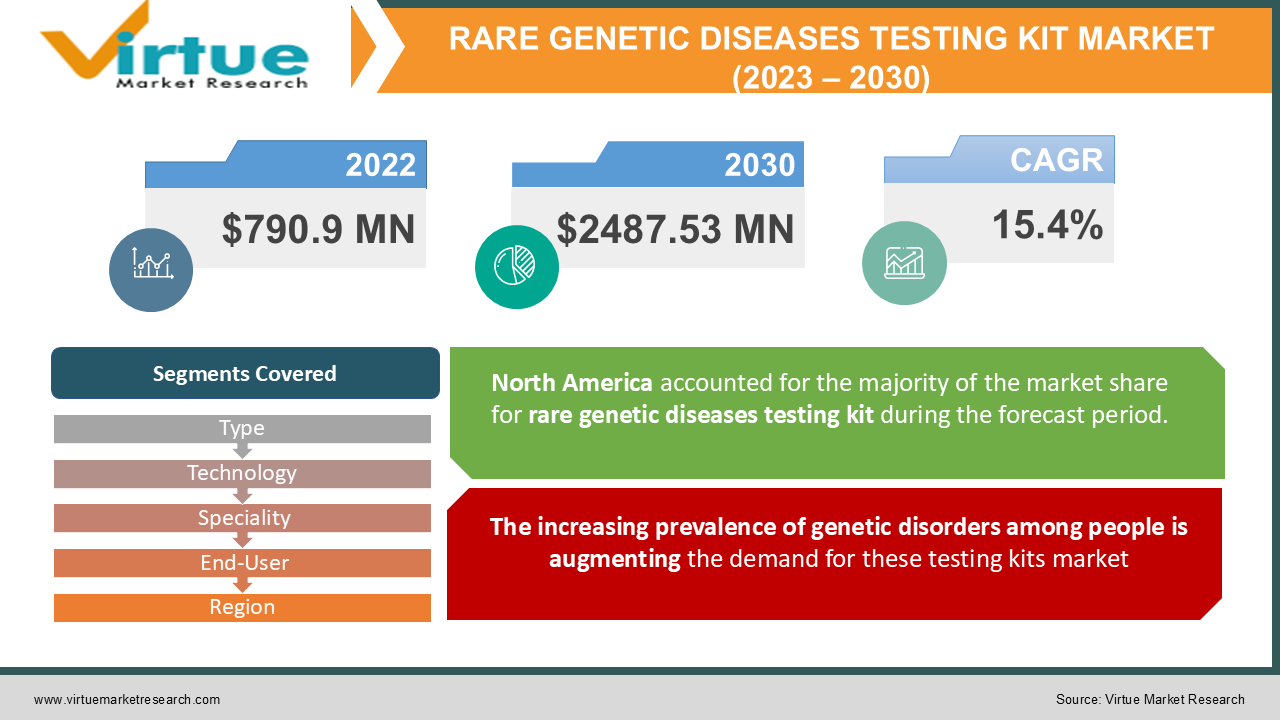
The global Rare Genetic Diseases Testing Kit market size is anticipated to be at USD 790.9 Million in 2022 to USD 2487.53 Million in 2030 and exhibit a CAGR of 15.4% during the forecast period, 2023-2030.
Market Overview:
Genetic testing is a medical test that spots changes in genes, chromosomes, and proteins. Theoutput of a genetic test can confirm or rule out a suspected genetic disease or condition or help indetermining the chances of developing or passing on a genetic disorder through the family tree.Almost 77,000 genetic tests are currently in use, and others are being developed due to their high demand among people. The global rare genetic diseases testing kit market is primarily driven by the increasing prevalence of genetic disorders, advancements in the technological department associated with the genetic testing market, and the surging capital investments in research and development in the industry by the leading players in the market. At the present time, healthcare expenditure has risen across several developed and developing nations due to the awareness programs initiated by their respective governments and authorities in the region. These are anticipated to create a competitive edge for manufacturers to develop and introduce new and enhanced genetic testing kits in the market in the forthcoming years.
COVID-19 Impact on the Rare Genetic Diseases Testing Kit Market:
The COVID-19 pandemic emerged in China in December 2019, and since then, it has traversedglobally at a very rapid pace. China, Iran, Italy, Spain, the Republic of Korea, Germany, France,and the US are among the worst-affected countries in terms of the number of deaths reported.The COVID-19 outbreak has critically influenced industries in several countries due to lockdowns and shutdowns. The global healthcare sector faced serious disturbances such as supply chain disruptions, technology events, scarcity of employees and laborers, and office shutdowns as a result of this outbreak. It disturbed the overall growth in three major ways: bydisrupting production and demand, its financial hit on the manufacturing industry, and by supply-chain disruptions. Additionally, patients with undiagnosed rare diseases have also beenfacing several health challenges. These challenges include diagnostic and pro-diagnostic uncertainties intertwined with medical complexities resulting in poor health outcomes. Clinal trials have also been affected by the COVID-19 pandemic. It created several restrictions for clinical trials challenging the findings, recruitment, and retaining patients with rare disease conditions.
Market Drivers:
The increasing prevalence of genetic disorders among people is augmenting the demand for these testing kits market:
The rising cases of genetic disorders and the associated complication with it among people causesevere health problems that may be incompatible with life. According to various reports, genetic disorders and congenital abnormalities are as common as about 2% - 5% of all live births andaccount for around 30% of paediatric admissions in hospitals. Therefore, the increase in thenumber of these cases is driving the demand for rare genetic disease testing kits in the global market.
Increasing adoption of advanced technologies and surging R&D investments are boosting the market size:
The evolvement of next-generation sequencing (NGS), especially for the treatment of cancer,and various other chronic diseases shift the focus toward genomics-centric pharmacology. NSGprovides advantages such as precise accuracy, sensitivity, and speed compared to conventional methods and makes impactful changes to the field of oncology. Continuous scientific innovations have created new opportunities for the development of technologies that impact themanagement of disorders. Collaborations are being undertaken with various stakeholders involved, such as researchers, patients, and regulators, for rare disease product development. Increasing health awareness among people is driving the healthcare industry and
Propelling the rare genetic disease testing kit market:
An increase in the levels of health awareness among people has resulted in a rise in the numberof available registries and is one of the major driving forces of the industry. It enables a pool ofdata of a sufficient sample size for epidemiological and clinical research. They play an importantrole in evaluating the feasibility of clinical trials and thus enabling the effective planning ofclinical trials and the enrolment process. Registries speed up service planning along withsupporting public health and clinical research by providing necessary insights to researchers.Government initiatives and strategies supporting the developments in the industry arefostering the market size growth:The strategic government initiatives for new product development in rare disease diagnoses further augment the market growth. These initiatives incorporate products for the diagnosis, advancement of educational awareness, working towards the enlargement of research capitals inrare diseases, and opening the doors for expert societies with an aim of refining rare undiagnosed genetic disease patient care and supervision thus, expanding the market size globally.
Market Restraints:
The high installation cost associated with the devices and R&D in genetic testing ispredicted to hamper the global market growth: The high and plummeting costs associated with sequencing and genetic testing are likely to slowdown the growth of the global rare diseases testing kit market. Also, establishing a certain andspecific diagnosis for a genetic disorder is a major challenge in the healthcare sector and henceincurs a significant amount of cost to detect and diagnose it, de-escalating the market growth rate.This research report on the Rare Genetic Diseases Testing Kit Market has been segmentedand sub-segmented based on Type, Technology, Speciality, End-User, Region, and Companies.
RARE GENETICS DISEASES TESTING KIT MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2022 - 2030 |
|
Base Year |
2022 |
|
Forecast Period |
2023 - 2030 |
|
CAGR |
15.4% |
|
Segments Covered |
By Type, Technology, Speciality, End-User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Thermo Fisher Scientific Inc., Invitae Corporation, Bio-Rad Laboratories, Inc. Perkin Elmer Inc., Illumina Inc .QIAGEN, F. Hoffmann-La Roche Ltd., Fulgent Genetics Myriad Genetics, Inc.Abbott, Eurofins Scientific, Sorenson Genomics, BIO-HELIX Biocartis, Cepheid (A subsidiary of Danaher) |
Rare Genetic Diseases Testing Kit Market – By Type.
-
Diagnostic Testing
-
Prenatal Testing
-
New Born Screening
-
Predictive and Pre symptomatic Testing
-
Carrier Testing
-
Other Types
Based on type, the rare genetic diseases testing kit market has been segmented into 6 segments –Diagnostic Testing, Prenatal Testing, New Born Screening, Predictive and Pre symptomatic Testing, Carrier Testing, and Others.
Rare Genetic Diseases Testing Kit Market – By Technology
-
Polymerase Chain Reaction
-
DNA Sequencing (NGS-Based Testing)
-
Whole Genome Sequencing
-
Microarrays
-
Fluorescence In Situ Hybridization (FISH)
-
Others
Based on technology, the rare genetic diseases testing kit market has been segmented into 6segments – Polymerase Chain Reaction, DNA Sequencing (NGS-Based Testing), WholeGenome Sequencing, Microarrays, Fluorescence in Situ Hybridization (FISH), and Others.The Next-Generation Sequencing (NGS) technology segment holds the maximum revenue shareof around 35% and dominates the segment in 2022. Wide-scale availability and rapid adoption ofNGS-based gene panels for cancer, neurologic disease, cardiovascular disease, paediatricconditions, and other genetic-related disease testing have driven the segment. Strategic initiatives by major players are anticipated to further augment the segment over the forecasted timeline.
Rare Genetic Diseases Testing Kit Market – By Speciality
-
Molecular Genetic Tests
-
Chromosomal Genetic Tests
-
Biochemical Genetic Tests
Based on speciality, the rare genetic diseases testing kit market has been segmented into 3 segments: Molecular Genetic Tests, Chromosomal Genetic Tests, and Biochemical GeneticTests. The molecular genetic tests segment dominates the global industry in 2022 and accounts for themaximum share of more than 41.00% of the global revenue. The segment will continue to retain its dominant position growing at the highest CAGR during the forecast period, 2023-2028. Anincrease in technological advancements and competence in handling & managing high-output ttechnologies within clinical arrangements are factors predicted to boost the segment growth.Furthermore, genome sequencing is the most advanced and unbiased testing method that is easilyavailable for both research and clinical purposes. This is primarily causing the declining costs ofsequencing tests along with the rapid development of next-generation sequencing-based new tests.
Rare Genetic Diseases Testing Kit Market – By End-User
-
Hospitals
-
Clinics
-
Diagnostic Centers
-
Private Clinics
-
Laboratory Service Providers
-
Private Laboratories
Based on the end-user, the rare genetic diseases kit market has been segmented into 6 segments –Hospitals, Clinics, Diagnostic Centers, Private Clinics, Laboratory Service Providers, and Private Laboratories. The research laboratories segment is leading the global market in 2022 and accounts for the highest share of more than 46.90% of the overall revenue. They offer solutions based on various specialties, including molecular genetic tests, chromosomal genetic tests, and biochemical genetic tests.The diagnostic laboratories segment is anticipated to witness the fastest CAGR over the forecast period owing to the rising number of mergers and collaboration activities of diagnosticlaboratories with genetic testing companies.
Rare Genetic Diseases Testing Kit Market – By Region
-
North America
-
Europe
-
Asia-Pacific
-
South America
-
Middle East and Africa
Based on regions, the rare genetic diseases testing kit market has been segmented into 5 major regions – North America, Europe, Asia-Pacific, South America, Middle-East, and Africa. North America is the major dominating region in the market in 2022, due to the increasing prevalence of genetic disorders amongst the population in the region. They are dominating therare genetic diseases testing kit market in terms of overall market share and market revenue and will continue to exert their dominance during the forecast timeline. This is due to genetic defects and chromosomal abnormalities in the population and rapid research and development processesboosting the market.
Rare Genetic Diseases Testing Kit Market – By Companies
-
Thermo Fisher Scientific Inc.
-
Invitae Corporation
-
Bio-Rad Laboratories
-
Inc .Perkin Elmer Inc.
-
Illumina Inc .QIAGEN
-
F. Hoffmann-La Roche Ltd.
-
Fulgent Genetics Myriad Genetics
-
Inc.Abbott Eurofins Scientific
-
Sorenson Genomics
-
BIO-HELIX Biocartis
-
Cepheid (A subsidiary of Danaher)
Recent key industry developments:
-
In June 2022, the U.S. FDA launched a 5-year strategy program for Rare Neurodegenerative Diseases, extending and improving the lives of people suffering fromrare diseases, by enabling patient access to innovative treatments.
-
In Feb 2022, Bionano Genomics launched an initiative, Rare Undiagnosed Genetic
-
Disease (RUGD) to help raise the level of dedication and focus in clinical and
-
translational research.
-
In December 2021, Thermo Fisher Scientific Inc., acquired PPD, Inc., a key global
-
supplier of clinical research services.
-
In June 2022, Avesthagen Ltd. formed a strategic partnership with Wipro Ltd. for the
-
popularization of its genetic testing offerings.
-
In November 2021, Genomenon and Alexion, AstraZeneca Rare Disease entered a
-
strategic partnership to make important information for the treatment and diagnosis of
-
rare diseases more easily accessible.
Chapter 1. RARE GENETIC DISEASES TESTING KIT MARKET – Scope & amp; Methodology
1.1. Market Segmentation
1.2. Assumptions
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2. RARE GENETIC DISEASES TESTING KIT MARKET –Executive Summary
2.1. Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-19 Impact Analysis
2.3.1. Impact during 2023 - 2030
2.3.2. Impact on Supply – Demand
Chapter 3. RARE GENETIC DISEASES TESTING KIT MARKET –Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4. RARE GENETIC DISEASES TESTING KIT MARKET -Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatory Scenario - By Region
4.3 Customer Analysis
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. RARE GENETIC DISEASES TESTING KIT MARKET - Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6. RARE GENETIC DISEASES TESTING KIT MARKET – By Type
6.1 Diagnostic Testing
6.2 Prenatal Testing
6.3 New Born Screening
6.4 Predictive and Pre symptomatic Testing
6.5 Carrier Testing
6.6 Other Types
Chapter 7. RARE GENETIC DISEASES TESTING KIT MARKET – By Technology
7.1 Polymerase Chain Reaction
7.2 DNA Sequencing (NGS-Based Testing)
7.3 Whole Genome Sequencing
7.4 Microarrays
7.5 Fluorescence In Situ Hybridization (FISH)
7.6 Others
Chapter 8. RARE GENETIC DISEASES TESTING KIT MARKET – By Speciality
8.1 Molecular Genetic Tests
8.2 Chromosomal Genetic Tests
8.3 Biochemical Genetic Tests
Chapter 9. Rare Genetic Diseases Testing Kit Market – By End-User
9.1 Hospitals
9.2 Clinics
9.3 Diagnostic Centers
9.4 Private Clinics
9.5 Laboratory Service Providers
9.6 Private Laboratories
Chapter 10. RARE GENETIC DISEASES TESTING KIT MARKET – By Region
10.1 North America
10.2 Europe
10.3 Asia-Pacific
10.4 South America
10.5 Middle East and Africa
Chapter 11. RARE GENETIC DISEASES TESTING KIT MARKET – By Companies
11.1 Thermo Fisher Scientific Inc.
11.2 Invitae Corporation
11.3 Bio-Rad Laboratories
11.4 Inc .Perkin Elmer Inc.
11.5 Illumina Inc .QIAGEN
11.6 F. Hoffmann-La Roche Ltd.
11.7 Fulgent Genetics Myriad Genetics
11.8 Inc.Abbott Eurofins Scientific
11.9 Sorenson Genomics
11.10 BIO-HELIX Biocartis
11.11 Cepheid (A subsidiary of Danaher)
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The global Rare Genetic Diseases Testing Kit market size is anticipated to be at USD 790.9 Million in 2022 to USD 2487.53 Million in 2030 and exhibit a CAGR of 15.4% during the forecast period, 2023-2030.
The global rare disease genetic diseases testing kit market is anticipated to grow at a CAGR of 15.4% from 2023 to 2030.
Next Generation Sequencing (NGS) dominates the testing kit market with a revenue share of 35% in 2023.
Some major players operating in the rare genetic diseases testing market include Quest Diagnostics, Perkin Elmer Inc., ARUP Laboratories, and Centogene.
Key factors that are fuelling the rare genetic diseases testing kit market growth include a crash in sequencing cost, growing patient registry for rare disease, & launch of new products and programs in rare genetic disease diagnosis