Rapid Diagnostics for Paramyxo Viruses Market Size (2024 – 2030)
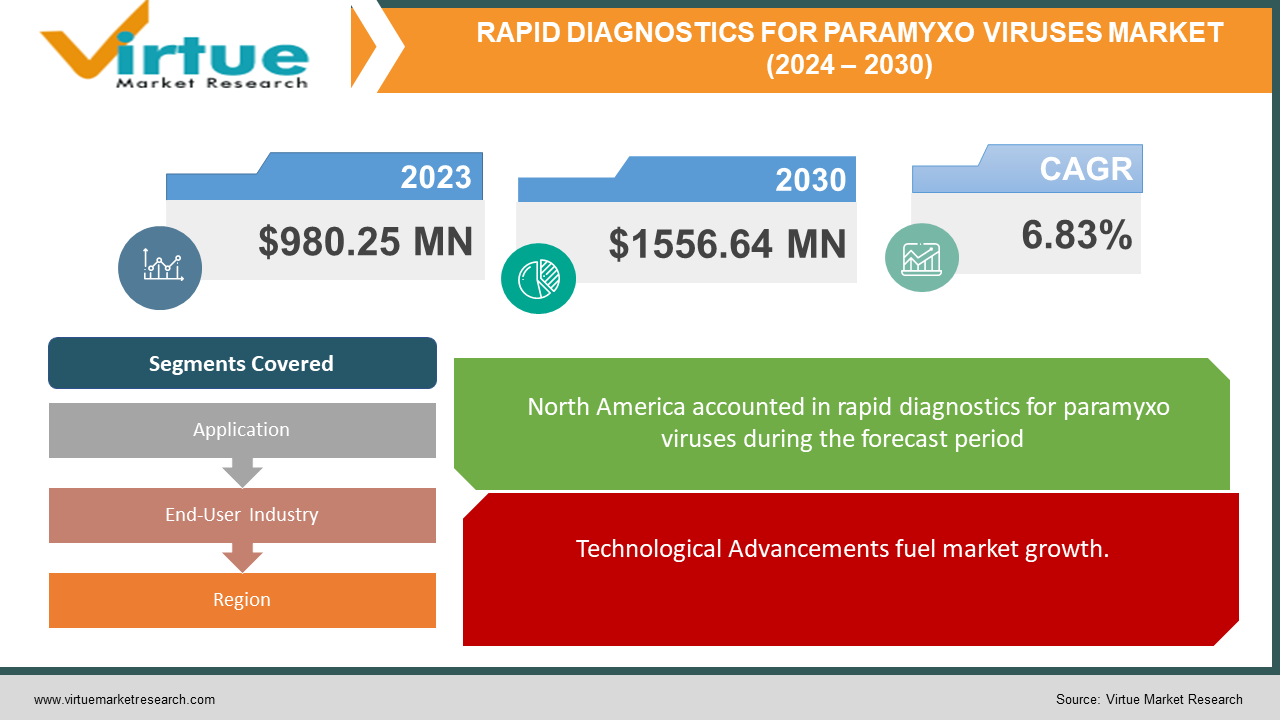
The Global Rapid Diagnostics for Paramyxo Viruses Market was valued at USD 980.25 million in 2023 and is projected to reach a market size of USD 1556.64 million by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 6.83%.
Parmaxyo's rapid diagnosis of diseases represents a significant advance in the management of infectious diseases and enables rapid and accurate detection. These tests use new technologies such as nucleic acid amplification testing (NAAT) and antigen-based detection to rapidly detect the presence of the virus within minutes. NAAT, which includes polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP), can detect genetic diseases with specificity and sensitivity and provide insight into pain management decisions. Antigen-based tests, on the other hand, directly detect infectious agents in patient samples and provide rapid results without the need for laboratory setup. These rapid diagnoses allow doctors to quickly begin appropriate treatment, take preventative measures, and prevent further spread. They also play an important role in public health services by facilitating the early detection and prevention of epidemics. With their ability to deliver timely and accurate results, rapid diagnostics for Parmaxyo viruses are indispensable tools in combating infectious diseases and safeguarding public health.
Key Market Insights:
North America region has the largest and had almost USD 365 million of total market share in 2023 and is expected to show a CAGR of 8.82%.Stringent regulatory standards and requirements govern the approval and commercialization of rapid diagnostic tests for Paramyxo viruses, influencing market entry strategies and product development efforts by companies operating in this space. The market is characterized by intense competition among key players, including multinational corporations, diagnostic companies, and emerging startups, who are continuously innovating and expanding their product portfolios to gain a competitive edge in the market.
Rapid Diagnostics for Paramyxo Viruses Market Drivers:
Technological Advancements fuel market growth.
Continuous advancements in diagnostic technologies play a pivotal role in driving the global rapid diagnostics market for Parmaxyo viruses. Developments such as point-of-care testing devices, miniaturization of diagnostic tools, and the integration of advanced molecular techniques like PCR and LAMP contribute to improved sensitivity, specificity, and quick diagnosis. These technological advancements improve the efficiency and accuracy of rapid diagnostics, making them indispensable tools for medical professionals in detecting Parmaxyo viruses rapidly.
Increasing the Prevalence of Parmaxyo Viruses accelerates the market growth.
The rising prevalence of Parmaxyo viruses globally is a significant driver fueling the demand for rapid diagnostics. With an increase in global travel, urbanization, and climate alteration, the risk of viral outbreaks and epidemics has escalated. Rapid diagnostics provides a timely and effective means of detecting these viruses, allowing early intervention, containment measures, and putting a stop to further spread. The growing awareness among healthcare providers and policymakers regarding the significance of early detection further propels the need for rapid diagnostic tests for Parmaxyo viruses.
Increasing Investments by Key Market Players will drive the Rapid Diagnostics for the Paramyxo Viruses market forward.
The rapid diagnostics market for Parmaxyo viruses is characterized by crucial investments and collaborations among key market players, including diagnostic equipment manufacturers, pharmaceutical firms, and research institutions. These investments are directed toward the development of innovative diagnostic platforms, expansion of product range, and strategic collaborations for distribution/sales and market penetration. Furthermore, mergers and acquisitions enable companies to establish their market presence, leverage complementary technologies, and improve their competitive position in the rapidly evolving landscape of rapid diagnostics for Parmaxyo viruses.
Rapid Diagnostics for Paramyxo Viruses Market Restraints and Challenges:
Limited Regulatory Approval and Standardization restrain the market growth.
One primary challenge in the Global Rapid Diagnostics Market for Parmaxyo viruses is the regulatory approval process and the lack of standardized testing protocols. Obtaining regulatory approval for rapid diagnostic tests requires a lot of time and is expensive, which in turn delays market entry for new products. Moreover, variations in regulatory requirements across different areas can create barriers to market penetration and increase compliance costs for manufacturers. The absence of standardized testing protocols may also lead to inconsistencies in test performance and interpretation, undermining the reliability and trustworthiness of rapid diagnostics for Parmaxyo viruses.
Accuracy and Reliability Concerns prove to be a challenge in the Rapid Diagnostics for Paramyxo Viruses Market.
Despite technological advancements, there are concerns regarding the accuracy and reliability of rapid diagnostic tests for Parmaxyo viruses. False-positive or false-negative results can have consequences such as misdiagnosis, inappropriate treatment, and the spread of infection. Factors such as specimen quality, assay design, and user proficiency can influence the performance of rapid diagnostic tests, leading to uncertainty in results. Ensuring the accuracy and reliability of these tests remains a critical challenge for manufacturers and healthcare providers, requiring ongoing validation studies, quality control measures, and user training programs.
Supply Chain Disruptions and Logistics Challenges hinder market growth.
The global rapid diagnostics market for Parmaxyo viruses is susceptible to supply chain disruptions and logistics challenges, particularly during public health emergencies such as pandemics or outbreaks. Limited availability of raw materials, reagents, and components can impact production capacity and lead to delays in product distribution/sales. Moreover, logistical constraints such as transportation bottlenecks, customs delays, and storage requirements can hinder the timely delivery of rapid diagnostic tests to end-users, particularly in resource-limited settings or remote areas. Addressing supply chain issues and improving logistics infrastructure is essential for ensuring the availability and accessibility of rapid diagnostics for Parmaxyo viruses during crises.
Rapid Diagnostics for Paramyxo Viruses Market Opportunities:
In the Global Rapid Diagnostics for Parmaxyo Viruses Market, several market opportunities pave the way for substantial growth and innovation. Firstly, the increasing focus on point-of-care testing and decentralized healthcare delivery presents an opportunity for rapid diagnostic manufacturers to develop handy, user-friendly testing devices that can be deployed in various healthcare settings, including remote areas and resource-limited settings. Secondly, advancements in digital medical technologies, such as mobile-based diagnostics and telemedicine platform services, offer novel avenues for improving the accessibility and efficiency of rapid diagnostic testing for Parmaxyo viruses. These digital solutions enable instant data collection, remote consultation, and seamless integration with medical systems, empowering healthcare providers to diagnose and manage infections more effectively. Moreover, the growing demand for rapid diagnostics in emerging markets, coupled with rising investments in healthcare infrastructure and disease surveillance, creates an environment for market expansion and penetration, driving the development of innovative diagnostic solutions.
RAPID DIAGNOSTICS FOR PARAMYXO VIRUSES MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
6.83% |
|
Segments Covered |
By Application, End-User Industry, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Abbott Laboratories, Roche Diagnostics, Thermo Fisher Scientific, bioMérieux, QuidelOrtho Corporation, Cepheid (Danaher Corporation), Bio-Rad Laboratories, DiaSorin S.p.A., Sekisui Diagnostics, QIAGEN |
Rapid Diagnostics for Paramyxo Viruses Market Segmentation - by Application
-
Respiratory Viruses
-
Gastrointestinal Viruses
-
Vector-Borne Viruses
-
Blood-Borne Viruses
-
Emerging and Zoonotic Viruses
In 2023, based on the Application, Respiratory Viruses hold the largest market share with over 70% of the market. Tests for respiratory viruses such as influenza and RSV are daily used in medical practice, especially during the winter months when these viruses are most active. Additionally, the emergence of new variants of respiratory viruses and the potential for future pandemics highlights the significance of rapid diagnostics in identifying and managing these infections quickly. Furthermore, advancements in technology have led to the development of multiplex assays that can diagnose multiple respiratory viruses simultaneously, enhancing the efficiency and cost-effectiveness of testing.
Rapid Diagnostics for Paramyxo Viruses Market Segmentation - by End-User Industry
-
Hospitals and Clinics
-
Diagnostic Laboratories
-
Research Institutes and Academic Centers
-
Point-of-Care Settings
-
Pharmaceutical and Biotechnology Companies
In 2023, based on the End-User Industry, Diagnostics Laboratories hold a significant portion of the market share and are expected to grow at an 8.51% CAGR during the forecast period. Diagnostic laboratories typically offer a wider spectrum of diagnostic tests, including molecular assays, antigen tests, and serological tests for various viral pathogens. This diversity enables them to serve a variety of healthcare needs and adapt to changing testing requirements. With their expertise and specialization, diagnostic laboratories employ skilled professionals, including laboratory scientists, technicians, and pathologists, who specialize in conducting and interpreting diagnostic tests. Their expertise ensures the accuracy and reliability of test results, making diagnostic laboratories trusted entities in the healthcare community.
Additionally, diagnostic laboratories stick to strict regulatory standards and quality control considerations to ensure the accuracy, reliability, and safety of diagnostic tests. Compliance with regulatory requirements solidifies trust among healthcare providers and patients, reinforcing the dominance of diagnostic laboratories in the market. Many diagnostic laboratories also indulge in research and development activities to innovate new diagnostic technologies/devices, improve testing methodologies, and enhance diagnostic accuracy. Their contributions to innovation accelerate advancements in the field of rapid diagnostics for Parmaxyo viruses, further strengthening their dominant position in the market.
Rapid Diagnostics for Paramyxo Viruses Market Segmentation: Regional Analysis
-
North America
-
Asia-Pacific
-
Europe
-
South America
-
Middle East and Africa
In 2023 based on Region, North America has the largest market share, with over 35% market share. North America, particularly the United States, has a robust healthcare infrastructure, high healthcare investments, and strong regulatory frameworks that facilitate the rapid adoption of new diagnostic technologies. The region is home to several established diagnostic companies and research institutions that fuel innovation in rapid diagnostics for Parmaxyo viruses. Additionally, the United States has been at the forefront of the COVID-19 pandemic response, accelerating significant demand for rapid diagnostic tests for SARS-CoV-2 and other respiratory viruses. Additionally, North America benefits from a high level of citizen awareness regarding infectious diseases and the importance of early diagnosis and intervention. This awareness, with a strong emphasis on preventive healthcare, contributes to the widespread use of rapid diagnostic tests for infections in clinical settings and public health surveillance programs
In Asia-Pacific, the market is propelled by robust healthcare infrastructure, technological advancements, and the prevalence of infectious diseases. Countries such as China, Japan, and India are driving economic growth through investments in healthcare and the increasing need for rapid diagnostic solutions. In Europe, stringent regulatory standards, complex healthcare systems, and a focus on disease surveillance contribute to the sector. Thanks to awareness of the disease and advances in treatment, rapid diagnosis is increasing in South America. In the Middle East and Africa, the market is characterized by efforts to improve disease control and management, driving demand for rapid diagnosis despite challenges in healthcare access.
While North America leads the Global Rapid Diagnostics for Paramyxo Viruses market, other regions such as Asia-Pacific and Europe are experiencing rapid growth and present huge opportunities for Rapid Diagnostics for Paramyxo Viruses market vendors.
COVID-19 Impact Analysis on the Global Rapid Diagnostics for Paramyxo Viruses Market:
The COVID-19 pandemic has had a major impact on the Global Rapid Diagnostics Paramyxo Viruses Market, creating both challenges and opportunities. While the pandemic has increased demand for rapid diagnosis and new diagnostic technologies, it has also disrupted the supply chain, delayed regulatory approvals, and strained medical resources. The demand for rapid diagnosis and treatment for COVID-19 has impacted economic growth, outpacing attention and resources for other infectious diseases. However, the pandemic also highlighted the importance of rapid diagnosis in disease surveillance and control, driving investment and development in the field for Paramyxo viruses and other infectious diseases.
Latest Trends/ Developments:
The Global Rapid Diagnostics for Paramyxo Viruses Market is witnessing several trends and developments shaping its course. One notable trend is the increasing adoption of point-of-care (POC) testing solutions, driven by the demand for rapid and decentralized diagnostic capabilities. POC tests offer quick results, enabling quick clinical decision-making and improving patient outcomes. Another emerging trend is the integration of advanced technologies such as molecular diagnostics, antigen detection, and immunochromatography in rapid diagnostic platform services. These technologies improve the sensitivity, specificity, and speed of diagnostic tests for Paramyxo viruses, highlighting the need for accurate and efficient diagnostics. Additionally, there is a rising focus on the development of multiplex assays capable of detecting various viral pathogens simultaneously, streamlining testing workflows and enhancing diagnostic efficiency.
Additionally, there is a growing emphasis on the development of user-friendly, portable, and cost-effective diagnostic solutions/devices to expand access to rapid testing in resource-limited regions. Overall, these trends reflect the developing course of rapid diagnostics for Paramyxo viruses, fueled by advancements in technology, changing healthcare needs, and efforts to enhance diagnostic performance and accessibility.
Key Players:
-
Abbott Laboratories
-
Roche Diagnostics
-
Thermo Fisher Scientific
-
bioMérieux
-
QuidelOrtho Corporation
-
Cepheid (Danaher Corporation)
-
Bio-Rad Laboratories
-
DiaSorin S.p.A.
-
Sekisui Diagnostics
-
QIAGEN
Chapter 1. RAPID DIAGNOSTICS FOR PARAMYXO VIRUSES MARKET – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. RAPID DIAGNOSTICS FOR PARAMYXO VIRUSES MARKET – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. RAPID DIAGNOSTICS FOR PARAMYXO VIRUSES MARKET – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. RAPID DIAGNOSTICS FOR PARAMYXO VIRUSES MARKET - Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. RAPID DIAGNOSTICS FOR PARAMYXO VIRUSES MARKET – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. RAPID DIAGNOSTICS FOR PARAMYXO VIRUSES MARKET – By Application
6.1 Introduction/Key Findings
6.2 Respiratory Viruses
6.3 Gastrointestinal Viruses
6.4 Vector-Borne Viruses
6.5 Blood-Borne Viruses
6.6 Emerging and Zoonotic Viruses
6.7 Y-O-Y Growth trend Analysis By Application
6.8 Absolute $ Opportunity Analysis By Application, 2024-2030
Chapter 7. RAPID DIAGNOSTICS FOR PARAMYXO VIRUSES MARKET – By End-User Industry
7.1 Introduction/Key Findings
7.2 Hospitals and Clinics
7.3 Diagnostic Laboratories
7.4 Research Institutes and Academic Centers
7.5 Point-of-Care Settings
7.6 Pharmaceutical and Biotechnology Companies
7.7 Y-O-Y Growth trend Analysis By End-User Industry
7.8 Absolute $ Opportunity Analysis By End-User Industry, 2024-2030
Chapter 8. RAPID DIAGNOSTICS FOR PARAMYXO VIRUSES MARKET , By Geography – Market Size, Forecast, Trends & Insights
8.1 North America
8.1.1 By Country
8.1.1.1 U.S.A.
8.1.1.2 Canada
8.1.1.3 Mexico
8.1.2 By Application
8.1.3 By End-User Industry
8.1.4 Countries & Segments - Market Attractiveness Analysis
8.2 Europe
8.2.1 By Country
8.2.1.1 U.K
8.2.1.2 Germany
8.2.1.3 France
8.2.1.4 Italy
8.2.1.5 Spain
8.2.1.6 Rest of Europe
8.2.2 By Application
8.2.3 By End-User Industry
8.2.4 Countries & Segments - Market Attractiveness Analysis
8.3 Asia Pacific
8.3.1 By Country
8.3.1.1 China
8.3.1.2 Japan
8.3.1.3 South Korea
8.3.1.4 India
8.3.1.5 Australia & New Zealand
8.3.1.6 Rest of Asia-Pacific
8.3.2 By Application
8.3.3 By End-User Industry
8.3.4 Countries & Segments - Market Attractiveness Analysis
8.4 South America
8.4.1 By Country
8.4.1.1 Brazil
8.4.1.2 Argentina
8.4.1.3 Colombia
8.4.1.4 Chile
8.4.1.5 Rest of South America
8.4.2 By Application
8.4.3 By End-User Industry
8.4.4 Countries & Segments - Market Attractiveness Analysis
8.5 Middle East & Africa
8.5.1 By Country
8.5.1.1 United Arab Emirates (UAE)
8.5.1.2 Saudi Arabia
8.5.1.3 Qatar
8.5.1.4 Israel
8.5.1.5 South Africa
8.5.1.6 Nigeria
8.5.1.7 Kenya
8.5.1.8 Egypt
8.5.1.9 Rest of MEA
8.5.2 By Application
8.5.3 By End-User Industry
8.5.4 Countries & Segments - Market Attractiveness Analysis
Chapter 9. RAPID DIAGNOSTICS FOR PARAMYXO VIRUSES MARKET – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
9.1 Abbott Laboratories
9.2 Roche Diagnostics
9.3 Thermo Fisher Scientific
9.4 bioMérieux
9.5 QuidelOrtho Corporation
9.6 Cepheid (Danaher Corporation)
9.7 Bio-Rad Laboratories
9.8 DiaSorin S.p.A.
9.9 Sekisui Diagnostics
9.10 QIAGEN
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The Global Rapid Diagnostics for Paramyxo Viruses Market was valued at USD 980.25 million in 2023 and is projected to reach a market size of USD 1556.64 million by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 6.83%.
The segments under the Global Rapid Diagnostics for Paramyxo Viruses Market by Application are Respiratory Viruses, Gastrointestinal Viruses, Vector-Borne Viruses, Blood-Borne Viruses, and Emerging and Zoonotic Viruses.
North America is the dominant region in the Global Rapid Diagnostics for Paramyxo Viruses Market.
Abbott Laboratories, Roche Diagnostics, Thermo Fisher Scientific, bioMérieux, QuidelOrtho Corporation, QIAGEN, etc.
The COVID-19 pandemic has had a major impact on the Global Rapid Diagnostics Paramyxo Viruses Market, creating both challenges and opportunities. While the pandemic has increased demand for rapid diagnosis and new diagnostic technologies, it has also disrupted the supply chain, delayed regulatory approvals, and strained medical resources. The demand for rapid diagnosis and treatment for COVID-19 has impacted economic growth, outpacing attention and resources for other infectious diseases. However, the pandemic also highlighted the importance of rapid diagnosis in disease surveillance and control, driving investment and development in the field for Paramyxo viruses and other infectious diseases.