Rapid Diagnostic Testing for Anal Cancer Market Size (2023 - 2030)
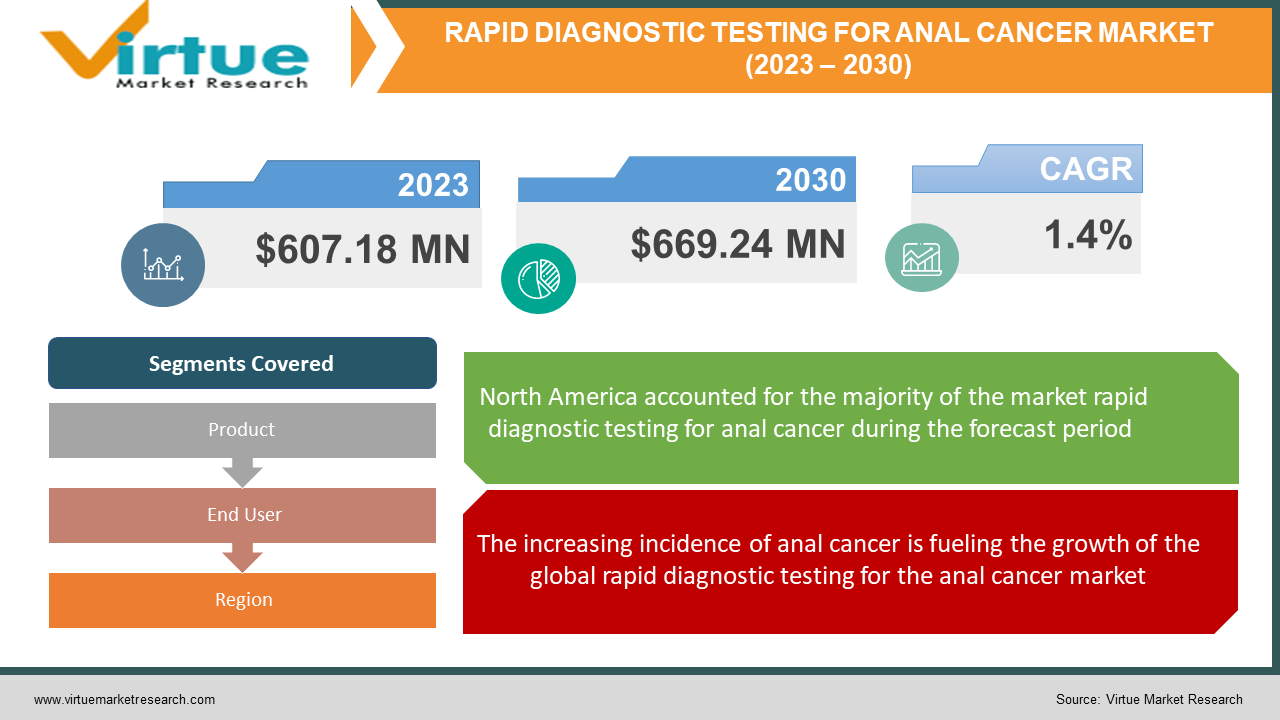
The Global Rapid Diagnostic Testing for Anal Cancer Market was valued at USD 607.18 million and is projected to reach a market size of USD 669.24 million by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 1.4%.
Anal cancer is a chronic illness in which malignant (cancer) cells develop in the tissues of the anus, the region of the body responsible for defecation. It lies below the rectum at the terminus of the large intestine and is comprised of both external skin layers and intestinal tissue. Sphincter muscles control the opening and closing of the anus. By keeping the anus closed, the internal sphincter muscle, an involuntary muscle, aids in continence maintenance. The external sphincter is a voluntary muscle that can be controlled consciously to open and close the anus. Mucus membranes that are covered with delicate nerve endings border the anal canal. This aids in detecting stools as they go through the anal canal. Primarily caused by HPV infection, other risk factors associated with anal cancer include weakened immunity, a particular family history of cancer, practicing receptive anal intercourse, low-risk sexual behavior, and smoking. Anal cancer signs also include bleeding from the anus or rectum, as well as the development of a mass near the anus. Pain or pressure around the anus, itching or discharge from the anus, and changes in bowel motions are also possible symptoms of anal cancer. Diagnosing anal cancer involves several procedures and tests. These include a digital rectal exam to check for abnormalities or growths, visual inspection using an anoscope, and ultrasound imaging to create detailed pictures of the anal canal. If any suspicious areas are found, a biopsy is performed to collect tissue samples for further examination under a microscope. Additional tests including CT scans, MRIs, and PET scans may be performed to identify the degree of the cancer and its dissemination. These imaging techniques provide detailed images of the body to evaluate the presence of cancer in nearby lymph nodes or other areas. The results of these tests help the doctor assign a stage to cancer using Roman numerals, indicating the size of the tumor and its spread beyond the anus. A higher stage signifies a more extensive spread of the cancer.
Global Rapid Diagnostic Testing for Anal Cancer Market Drivers:
The increasing incidence of anal cancer is fueling the growth of the global rapid diagnostic testing for the anal cancer market.
Anal cancer is generally diagnosed in those surpassing the age of 60 years, accounting for around 80% of instances. Men are more probable than women to get anal cancer under the age of 35, although women are slightly more susceptible past the age of 50. In contrast to married men, unmarried men have a much-increased chance of developing anal cancer, with a six-fold higher incidence witnessed in this demographic. Engaging in receptive anal intercourse is strongly correlated to the development of anal cancer. Contracting HPV infection, which can provoke genital warts, is a major risk element for anal cancer. Contrasted to individuals with potent immune systems, those with debilitated immune systems, such as HIV-positive individuals, generally have a less favorable prognosis and are more unguarded to anal cancer. Therefore, this factor drives the demand for rapid diagnostic testing services for anal cancer detection.
The growing demand for novel and effective diagnostic techniques is another factor contributing to the growth of the global rapid diagnostic testing for the anal cancer market.
The rising incidence of anal cancer among the population has created a growing need for the development of novel and effective diagnostic techniques. This need stems from the necessity to accurately and efficiently diagnose anal cancer to facilitate timely treatment and containment measures. As a result, the demand for rapid diagnostic testing services for anal cancer detection has witnessed a significant surge, fueling the growth of the market. The development of innovative diagnostic techniques that can offer improved accuracy, sensitivity, and rapid results becomes crucial in addressing the rising population diagnosed with anal cancer. Consequently, this increasing need for novel and useful diagnostic techniques acts as a market driver, propelling the growth of the global rapid diagnostic testing for the anal cancer market during the forecast period.
Global Rapid Diagnostic Testing for Anal Cancer Market Challenges:
The global rapid diagnostic testing for anal cancer market is encountering challenges, primarily in terms of the existence of strict regulations. The development of novel rapid diagnostic testing kits has been hindered by stringent regulatory norms, particularly in the United States where diagnostic products undergo a rigorous clearance process by the FDA. These regulatory barriers limit the introduction of innovative diagnostic testing products, which can potentially restrict the revenue growth of the market. Moreover, limitations imposed on certain testing kits by regulatory bodies can impede the progress and introduction of advanced and innovative diagnostic testing products. These challenges about regulatory policies and restrictions pose obstacles to the overall revenue growth of the market.
Global Rapid Diagnostic Testing for Anal Cancer Market Opportunities:
Market expansion strategies present lucrative opportunities in the global rapid diagnostic testing for the anal cancer market. Major players in the global rapid diagnostic testing market for anal cancer market have the potential for growth in developing economies like India, South Korea, Brazil, and Mexico. These countries provide opportunities owing to their high disease rates, large populations in need of healthcare, improved medical facilities, rising disposable incomes, and a growing trend of medical tourism. Additionally, the region of Asia-Pacific, including these countries, is becoming an attractive destination for business owing to its adaptable environment and fewer strict regulations and data requirements. This combination of factors makes these economies promising markets for companies in the rapid diagnostics sector.
COVID-19 Impact on the Global Rapid Diagnostic Testing for Anal Cancer Market:
The outbreak of the COVID-19 pandemic substantially impacted the global rapid diagnostic testing for the anal cancer market. The pandemic caused disruptions in supply chains and distribution of goods and services, which highly affected the supply of essential materials like reagents and testing equipment for rapid diagnostic testing for anal cancer. Moreover, the shifted focus of healthcare infrastructure resources and personnel to COVID-19 testing and treatment further declined the demand for rapid diagnostic testing for anal cancer. These factors negatively impacted the growth of the global rapid diagnostic testing for the anal cancer market. Despite these challenges, the global rapid diagnostic testing for anal cancer market is projected to recover and grow in the coming years.
Global Rapid Diagnostic Testing for Anal Cancer Market Recent Developments:
-
In April 2023, Biosynex SA completed its acquisition of Chembio Diagnostics, Inc., a renowned company focused on infectious disease diagnostic testing. Chembio is now a part of the Biosynex Group. The merger is anticipated to propel growth through the combination of their technologies and product portfolios, as well as the expanded market opportunities.
-
In February 2023, EXACT Sciences Corporation unveiled a new genomic test called OncoExTra for cancer therapy selection in the United States. This cutting-edge test analyzes both DNA and RNA utilizing next-generation sequencing (NGS) technology, offering clinicians a thorough molecular profile of the patient's malignancy. With personalized and actionable results, OncoExTra aims to provide individuals with tailored insights to guide their cancer treatment decisions.
-
In June 2022, A National Cancer Institute (NCI)-funded clinical trial uncovered that treating high-grade squamous intraepithelial lesions (HSIL) in HIV-positive minimizes the probability of anal cancer by over 50%. The study, involving 10,000+ participants, emphasized the significance of screening and treating anal HSIL in this population and potentially other high-risk groups. Additional research and clinician training is warranted. The trial also established a biorepository for future analysis of tissue samples.
RAPID DIAGNOSTIC TESTING FOR ANAL CANCER MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2022 - 2030 |
|
Base Year |
2022 |
|
Forecast Period |
2023 - 2030 |
|
CAGR |
1.4% |
|
Segments Covered |
By Product, End User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Biosynex SA (France), EXACT Sciences Corporation (United States), GY Highland Biotech LLC (United States), Hologic, Inc. (United States), Lepu Medical Technology (Beijing) Co., Ltd. (China), NTBIO Diagnostics Inc. (Canada), Hysen (Hangzhou) Biotech Co., Ltd. (China), Turklab Tibbi Malzemeler San. Tic. A.S. (Turkey), Xiamen Spacegen Co., Ltd. (China), Nanjing Liming Bio-products Co., Ltd. (United States) |
Global Rapid Diagnostic Testing for Anal Cancer Market Segmentation: By Product
-
Consumables
-
Antibodies
-
Kits & Reagents
-
Probes
-
Other Consumables
-
-
Instruments
-
Pathology-based Instruments
-
Imaging Instruments
-
Biopsy Instruments
-
In 2022, the consumables segment held the highest market share. The growth can be attributed to the increasing demand for testing devices that provide fast and accurate results, and consumables play a vital role in the diagnostic process. Consumables like reagents and kits are extensively used in research and clinical settings as they offer consistent and precise results. Additionally, these consumables are cost-effective, making them a preferred choice for healthcare facilities. The rising adoption of consumables contributes to the revenue growth of this segment.
Global Rapid Diagnostic Testing for Anal Cancer Market Segmentation: By End User
-
Academic & Research Institutions
-
Diagnostic Centers
-
Hospitals & Clinical Laboratories
-
Others
In 2022, the hospitals and clinical laboratories segment held the highest market share. The growth can be attributed to the high patient footfall in these healthcare settings. They offer comprehensive healthcare facilities, with dedicated laboratory facilities and trained medical professionals for efficient rapid diagnostic testing services. Hospitals also have critical care units and emergency departments for the timely intervention and management of anal cancer-diagnosed patients. Additionally, anal cancer-diagnosed individuals often require hospitalization, and hospitals provide a suitable environment for continuous monitoring and intervention. Access to resources, including advanced diagnostic equipment and skilled personnel, further enables hospitals and clinical laboratories to perform a variety of rapid diagnostic testing services efficiently.
Global Rapid Diagnostic Testing for Anal Cancer Market Segmentation: By Region
-
North America
-
Europe
-
Asia-Pacific
-
South America
-
Middle East & Africa
The region of North America held the largest share of the global rapid diagnostic testing for anal cancer market in the year 2022. The rising incidence of anal cancer among individuals, the presence of well-established healthcare infrastructure in nations, such as the United States and Canada, rapid advancements in clinical diagnostics, and the introduction of new and improved technologies that aid in the accurate and efficient detection of anal cancer are some of the factors propelling the region's growth. Additionally, North America is home to several significant market players, including EXACT Sciences Corporation, GY Highland Biotech LLC, Hologic, Inc., NTBIO Diagnostics Inc., and Nanjing Liming Bio-products Co., Ltd. Due to the rising development of the medical and diagnostics industry in nations, such as China and India, the growing awareness and emphasis on preventive healthcare, such as anal cancer screening and treatment, and the strong presence of major market players, including Lepu Medical Technology (Beijing) Co., Ltd., Hysen (Hangzhou) Biotech Co., Ltd., and Xiamen Spacegen Co., Ltd., the region of Asia-Pacific are anticipated to expand at the quickest rate over the forecast period.
Global Rapid Diagnostic Testing for Anal Cancer Market Key Players:
-
Biosynex SA (France)
-
EXACT Sciences Corporation (United States)
-
GY Highland Biotech LLC (United States)
-
Hologic, Inc. (United States)
-
Lepu Medical Technology (Beijing) Co., Ltd. (China)
-
NTBIO Diagnostics Inc. (Canada)
-
Hysen (Hangzhou) Biotech Co., Ltd. (China)
-
Turklab Tibbi Malzemeler San. Tic. A.S. (Turkey)
-
Xiamen Spacegen Co., Ltd. (China)
-
Nanjing Liming Bio-products Co., Ltd. (United States)
Chapter 1. Rapid Diagnostic Testing for Anal Cancer Market – Scope & Methodology
1.1. Market Segmentation
1.2. Assumptions
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2. Rapid Diagnostic Testing for Anal Cancer Market – Executive Summary
2.1. Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-19 Impact Analysis
2.3.1. Impact during 2023 - 2030
2.3.2. Impact on Supply – Demand
Chapter 3. Rapid Diagnostic Testing for Anal Cancer Market – Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4. Rapid Diagnostic Testing for Anal Cancer Market - Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatory Scenario - By Region
4.3 Customer Analysis
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. Rapid Diagnostic Testing for Anal Cancer Market - Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6. Rapid Diagnostic Testing for Anal Cancer Market - By Product
6.1 Consumables
6.1.1 Antibodies
6.1.2 Kits & Reagents
6.1.3 Probes
6.1.4 Other Consumables
6.2 Instruments
6.2.1 Pathology-based Instruments
6.2.2 Imaging Instruments
6.2.3 Biopsy Instruments
Chapter 7. Rapid Diagnostic Testing for Anal Cancer Market - By End User
7.1 Academic & Research Institutions
7.2 Diagnostic Centers
7.3 Hospitals & Clinical Laboratories
7.4 Others
Chapter 8. Rapid Diagnostic Testing for Anal Cancer Market - By Region
8.1 North America
8.2 Europe
8.3 Asia-Pacific
8.4 Rest of the World
Chapter 9. Rapid Diagnostic Testing for Anal Cancer Market - Key Players
9.1 Biosynex SA (France)
9.2 EXACT Sciences Corporation (United States)
9.3 GY Highland Biotech LLC (United States)
9.4 Hologic, Inc. (United States)
9.5 Lepu Medical Technology (Beijing) Co., Ltd. (China)
9.6 NTBIO Diagnostics Inc. (Canada)
9.7 Hysen (Hangzhou) Biotech Co., Ltd. (China)
9.8 Turklab Tibbi Malzemeler San. Tic. A.S. (Turkey)
9.9 Xiamen Spacegen Co., Ltd. (China)
9.10 Nanjing Liming Bio-products Co., Ltd. (United States)
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
Global Rapid Diagnostic Testing for Anal Cancer Market is estimated to be worth USD 598.80 Million in 2022 and is projected to reach a value of USD 669.25 Million by 2030, growing at a CAGR of 1.4% during the forecast period 2023-2030.
The Global Rapid Diagnostic Testing for Anal Cancer Market Drivers are the increasing incidence of anal cancer and the growing demand for novel and effective diagnostic techniques.
Based on the Product, the Global Rapid Diagnostic Testing for Anal Cancer Market is segmented into Consumables and Instruments
The United States and Canada are the most dominating countries in the region of North America for the Global Rapid Diagnostic Testing for Anal Cancer Market.
Biosynex, EXACT Sciences Corporation, and GY Highland Biotech LLC are the leading players in the Global Rapid Diagnostic Testing for Anal Cancer Market.



