GLOBAL PROSTATE CANCER TESTING KITS MARKET SIZE (2023 -2030)
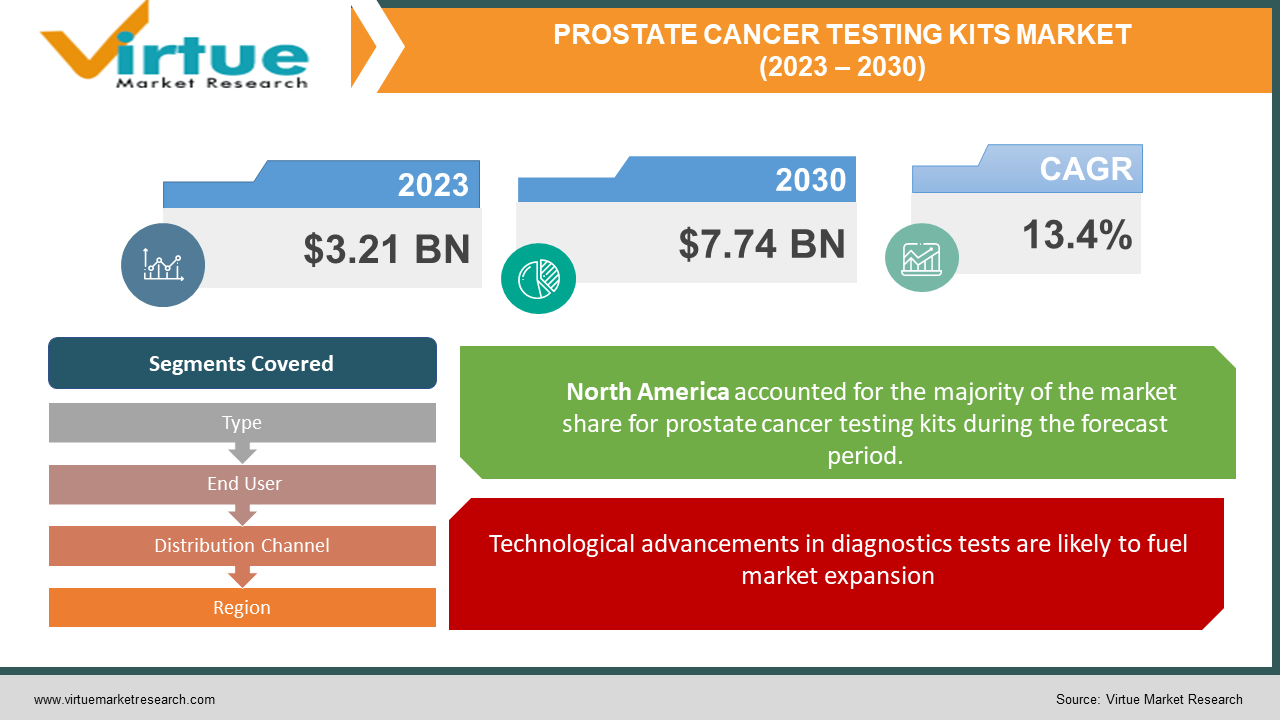
The Global Prostate Cancer Testing Kits Market was valued at USD 3.21 Billion and is projected to reach a market size of USD 7.74 Billion by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 13.4%.
INDUSTRY OVERVIEW
Diagnostics for prostate cancer are crucial to the early identification and effective treatment of the condition. Utilization of equipment, reagents, consumables, and accessories are all included. The most frequent first diagnosis for prostate cancer is PSA testing, which may or may not need follow-up tests to confirm the tumor’s presence. The use of prostate cancer diagnostics offers several advantages, including aiding in the early discovery of the disease at localized stages and assisting individuals in achieving effective health management. The usage of prostate cancer diagnostics is on the rise all around the world, but it is more prevalent in developing nations with rising healthcare standards. If the prostate cancer screening test results in a cancer diagnosis, further testing, such as a biopsy and PCA3 tests, may be necessary. MRI, transrectal ultrasonography (TRUS), or a combination of the two imaging methods are typically used by the clinician to examine the prostate during the biopsy. The worldwide market for prostate cancer diagnostics is expanding as a result of rising awareness of early detection and the availability of cutting-edge approaches in the industry.
COVD-19 IMPACT ON THE PROSTATE CANCER TESTING KITS MARKET
Worldwide, the COVID-19 pandemic has had an impact on several businesses. To slow the pandemic's rapid spread, governments all across the world enacted stringent lockdown regulations and social segregation standards. During the early phases of the epidemic, factories all across the world were shut down. Additionally, the economic downturn that followed the epidemic may cause a considerable delay in the commercial launch of the electronics sector. The backbone of technology providers, small and medium-sized businesses, has seen a sharp decline in income since the pandemic's debut in 2020. As a result of the supply chain interruptions, market participants faced several difficulties. However, when additional supplies come online in the second half of 2022, things will get better.
MARKET DRIVERS:
Technological advancements in diagnostics tests are likely to fuel market expansion
During the projected period, technological developments in diagnostic tests are anticipated to further fuel market expansion. Researchers are now experimenting with using artificial intelligence (AI) to identify prostate cancer, according to the National Cancer Institute. Artificial intelligence (AI) algorithms are used to spot worrisome regions in Magnetic Resonance Imaging (MRI) scans that need to be biopsied to confirm the presence of cancerous cells. Additionally, experiments using artificial intelligence technologies are being conducted to enhance biopsy sample analysis and assist the production of more effective and precise findings.
The increase in government support is fueling the market expansion
Positive market effects are anticipated as the government attempts to create new technology to improve diagnosis increase. For instance, the National Cancer Institute started the Prostate Specialized Programs of Research Excellence (SPORE) initiative, which aims to translate scientific discoveries into outcomes depending on clinical contexts. The institution also supports the creation of innovative technologies and research to improve monitoring, prevention, diagnosis, and treatment. Therefore, rising government initiatives and support are positively influencing market growth
New entrants in the market are promoting healthy competition which is acting as a growth factor
Another factor projected to fuel the growth of the prostate cancer diagnostics market shortly is the increasing number of new market entrants. To assist its attempts to create a unique at-home screening test, for instance, Gregor Diagnostics funded USD 9,00,000 in July 2018. In comparison to standard PSA testing, this gadget is anticipated to enable extremely sensitive test findings. As a result, it is anticipated that increased investment by new players would greatly fuel growth.
MARKET RESTRAINTS:
Lack of consumer knowledge will restrain market expansion for oncology diagnostics testing kit
The significance of clinical trials needs to be raised among patients, doctors, and the general public. Providing unique access to the most recent, reliable, and evidence-based information about these novel cancer medications can be useful in raising physicians’ awareness of them. Access to a widely accepted source of truth must be expanded if public awareness is to increase. Monthly periodicals and informational pamphlets are offered by numerous publishing firms and industry participants. To ensure that patients and healthcare professionals can access and comprehend the advantages of diagnostics, stakeholders have also concentrated on raising awareness through outreach program.
Due to the high initial cost, many end users cannot afford these testing kits, which could impede market expansion
Despite offering guaranteed returns on investment, the hefty initial cost puts these kits outside the means of a huge number of end users, particularly those in developing nations. Because they view the cost of using such diagnostic kits as a burden on their budgets, end-users like pharmaceutical companies, reference labs, hospitals, and CROs are prompted to choose third-party diagnostic services
PROSTATE CANCER TESTING MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2022 - 2030 |
|
Base Year |
2022 |
|
Forecast Period |
2023 - 2030 |
|
CAGR |
13.4 % |
|
Segments Covered |
By Type, End User, Distribution Channel and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
MDX HEALTH, MYRIAD GENETICS; INC., ABBOTT LABORATORIES, F. HOFFMAN-LA ROCHE AG, SIEMENS HEALTHINEERS AG OPKO HEALTH; INC., GENOMIC HEALTH. |
This research report on the Prostate Cancer Testing Kits Market has been segmented and sub-segmented based on Type, End-User, By Distribution Channel and By Region.
PROSTATE CANCER TESTING KITS MARKET– BY TYPE
-
Preliminary Tests
-
Confirmatory Tests
-
-
-
PCA3 Tests
-
Trans-rectal Ultrasound
-
Biopsy
-
-
-
Based on the type, the prostate cancer testing market is segmented into Preliminary tests and Confirmatory tests. Due to an increase in the frequency of prostate cancer, the preliminary test category is anticipated to have the biggest market share over the projection period. The wide accessibility of PSA screening has improved early identification and treatment, enhancing survival rates, claims the American Institute for Cancer Research. Unidentified and tiny tumors that may or may not progress to an advanced stage of cancer are found by PSA testing. Most men over 50 and those at risk of developing the condition are advised to get these tests since they enable identification before serious symptoms manifest. During the projection period, the Food and Drug Administration (FDA) is predicted to approve more products, which will accelerate market expansion. For instance, Cleveland Diagnostics Inc.'s IsoPSA Test, a cutting-edge non-invasive blood-based diagnostic assay for the identification of the condition, was granted FDA Breakthrough Device Designation in October 2019.
The Digital Rectal Exam (DRE) and biomarker exams are some more preliminary testing. DRE examinations are utilised to identify hard, lumpy, as well as abnormal prostate gland development. The accuracy of the findings acquired from these tests depends on the technician's knowledge and capabilities, thus further tests like PSA and biopsy are frequently needed. When males are having surgery, biomarkers can help identify aggressive malignancies, differentiate between significant and non-significant tumors, and identify them. These statistics demonstrate how public awareness is growing, making it easier to diagnose diseases early and treat them, which is boosting the market's expansion.
PCA3 test, Transrectal Ultrasound (TRUS), and biopsy are examples of confirmation testing. Patients must receive confirmatory tests for a more thorough diagnosis after preliminary testing if the PSA and DRE values are abnormal. The use of confirmatory tests has increased in response to growing illness prevalence, which is predicted to boost the market.
PROSTATE CANCER TESTING KITS MARKET- BY END-USER
-
Independent Diagnostic Laboratories
-
Hospitals
-
Cancer Research Institutes
-
Ambulatory Surgical Centers
-
Others
Based on the end-user, the prostate cancer testing market is segmented into independent diagnostic laboratories, hospitals, cancer research institutes, clinics, centres and others. Independent diagnostic laboratories, a group that specialises in performing different prostate cancer diagnostics, are currently leading the market for prostate cancer diagnostics in 2021. Additionally, independent diagnostic laboratories make it possible for consulting physicians and healthcare institutions to outsource a large number of diagnostic tests to them for better patient care. This results in robust, timely, cost-effective, and high-quality diagnostic care and services in a secure healthcare environment.
PROSTATE CANCER TESTING KITS MARKET- BY DISTRIBUTION CHANNEL
-
Direct tender
-
Retail Sales
Based on the type, the prostate cancer testing market is segmented into direct tender and retail sales. As the principal means through which healthcare institutions and diagnostic labs purchase reagents, consumables, equipment, and accessories, the direct tender segment will rule the prostate cancer diagnostics market in 2021. Healthcare service companies are concentrating on improving client services while also lowering total costs as a result of the rising expense of healthcare. For healthcare providers, direct tender product procurement is quite cost-effective.
PROSTATE CANCER TESTING KITS MARKET- BY REGION
-
North America
-
Europe
-
The Asia Pacific
-
Latin America
-
The Middle East
-
Africa
By region, the Prostate Cancer Testing Kits Market is grouped into North America, Europe, Asia Pacific, Latin America, The Middle East and Africa. Due to the high incidence of the condition and the growth in government measures to promote early detection, North America held the biggest market share in 2021. The National Cancer Institute, for instance, has put in place several program to improve research efforts for early diagnosis and treatment. One of their initiatives is the Cancer Biomarkers Research Group, which supports biomarker research. Similarly, the organization also oversees the Early Detection Research Network (EDRN), a consortium of federal and privately funded researchers working to identify and validate biomarkers for the detection of cancer. Additionally, throughout the forecast period, a rise in disease frequency in North America is anticipated to drive up demand for prostate cancer diagnostics. Prostate cancer is ranked as the second most frequent cancer in males in the United States, according to the American Institute for Cancer Research. In the U.S., more than 1.3 million instances of prostate cancer were identified in 2018. As a result, the market expansion is predicted to be fueled by the growing occurrence shortly.
Market expansion in Europe is anticipated to be fueled by expanding awareness-raising activities. The European Association of Urology launched the European Prostate Cancer Awareness initiative in 2020 to increase public awareness of early detection. Topics including overdiagnosis, overtreatment, and the effects of late discovery were discussed in the awareness program. Demand is also projected to be fueled by the increased prevalence in Europe. Prostate cancer affected more than 2 million men in Europe in 2018, and as a result, there were almost 92,000 annual fatalities, according to the European Association of Urology. The condition is estimated to cost US$10.6 billion annually, which demonstrates its expanding prevalence.
The National Institute of Health reports that the growth in disease epidemiology in Asian nations is caused by changes in lifestyle, such as a rise in the consumption of westernized foods and a decline in physical activity. Furthermore, approximately 297,215 people in Asia received prostate cancer diagnoses in 2020, according to information from the International Agency for Cancer Research. Thus, it is projected that increasing prevalence would further need the demand for diagnostic solutions, enabling significant regional expansion in the years to come.
PROSTATE CANCER TESTING KITS MARKET- BY COMPANIES
Some of the major players operating in the Prostate Cancer Testing Kits Market include:
1. MDX HEALTH
2. MYRIAD GENETICS; INC.
3. ABBOTT LABORATORIES
4. F. HOFFMAN-LA ROCHE AG
5. SIEMENS HEALTHINEERS AG
6. OPKO HEALTH; INC.
7. GENOMIC HEALTH.
NOTABLE HAPPENING IN THE PROSTATE CANCER TESTING KITS MARKET
- APPROVAL- OPKO Health, Inc. declared on December 8, 2021, that the FDA has authorized the use of OPKO's 4Kscore Test. Men over the age of 45 who have a high total PSA that is specific to their age and an abnormal digital rectal exam and have never had a prostate biopsy or who have had a biopsy but were negative may utilise this test.
- PRODUCT LAUNCH- In October 2020 Siemens Healthcare GmbH announced the introduction of the Biograph Vision Quadra Extended Axial FoV PET/CT Scanner. This scanner is the most sensitive extended field view scanner available and was created for clinical and academic use. The device will raise demand for cancer diagnostic procedures and will boost the company's income because of its extensive clinical use.
- PARTNERSHIP- In March 2020, TTP plc, a well-known independent technology and product development company, and DiaSorin S.p.A. confirmed that they had signed an exclusive licensing and technology transfer agreement. Through this contract, DiaSorin will have access to TTP's Puckdx TM, a versatile and affordable platform for the automation of sample-to-answer diagnostic assays. This will assist the company's diagnostic business profile expansion and will aid in the growth of its next ventures.
Chapter 1. AUTOMOTIVE GAN DEVICES MARKET – Scope & Methodology
1.1. Market Segmentation
1.2. Assumptions
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2. AUTOMOTIVE GAN DEVICES MARKET – Executive Summary
2.1. Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-19 Impact Analysis
2.3.1. Impact during 2023 - 2030
2.3.2. Impact on Supply – Demand
Chapter 3. AUTOMOTIVE GAN DEVICES MARKET – Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4. AUTOMOTIVE GAN DEVICES MARKET - Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatory Scenario - By Region
4.3 Customer Analysis
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. AUTOMOTIVE GAN DEVICES MARKET - Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6. AUTOMOTIVE GAN DEVICES MARKET – By Type
6.1. Preliminary Test
6.2. Confimentary Test
6.2.1. PCA3 Tests
6.2.2. Trans rectal Ultrasound
6.2.3. Biospy
Chapter 7. AUTOMOTIVE GAN DEVICES MARKET – By End User
7.1. Independent Diagnostics Laboratories
7.2. Hospitals
7.3. Cancer Research Institutes
7.4. Ambulatory Surgical Centers
7.5. Others
Chapter 8. AUTOMOTIVE GAN DEVICES MARKET – By Distribution Channel
8.1. Direct Tendor
8.2. Retail Sales
Chapter 9. PROSTATE CANCER TESTING KITS MARKET – By Region
9.1. North America
9.2. Europe
9.3. Asia-P2acific
9.4. Latin America
9.5. The Middle East
9.6. Africa
Chapter 10. PROSTATE CANCER TESTING KITS MARKET – By Companies
10.1. MDX HEALTH
10.2. MYRAID GENETICS LTD
10.3. ABBOTT LABORATORIES
10.4. F.HOFFMAN-LA ROCHE AG
10.5. SEIMENS HEALTHINEERS INC
10.6. OPKO HEALTH INC
10.7. GENOMIC HEALTH INC
Download Sample
Choose License Type
2500
4250
5250
6900