Progesterone Test for Endometrial Cancer Market Size (2024 – 2030)
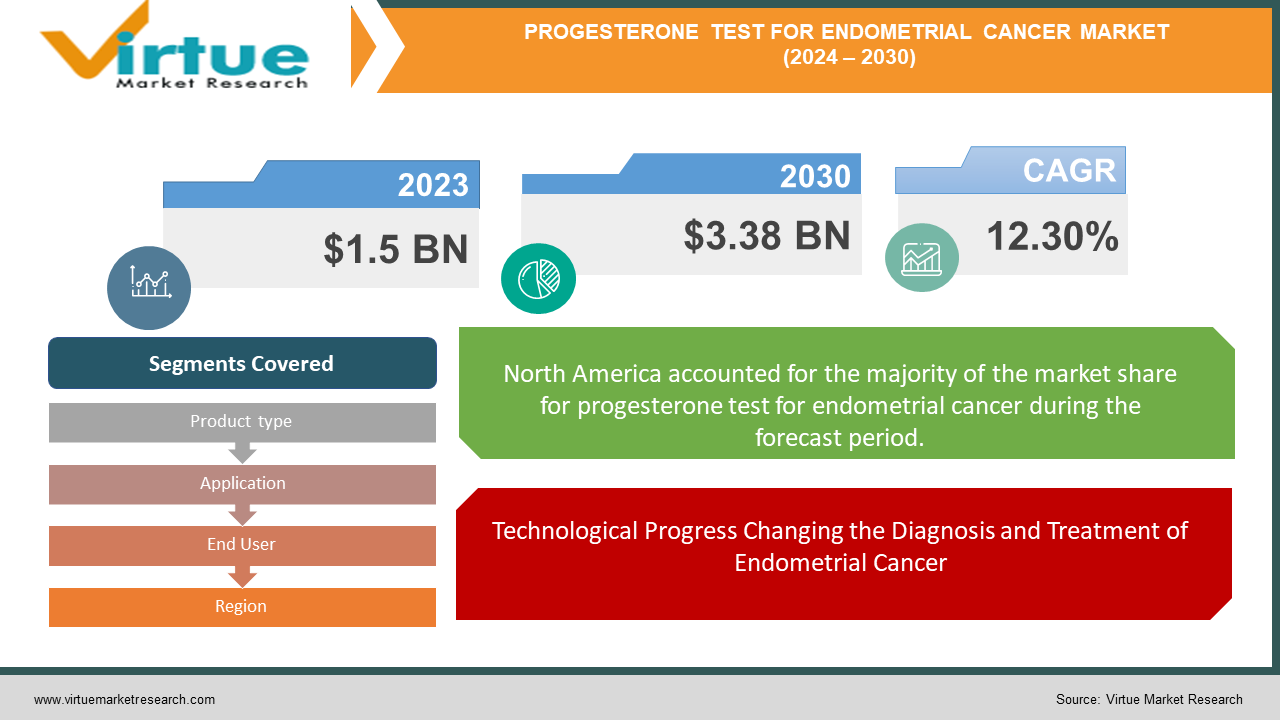
The Global Progesterone Test for Endometrial Cancer Market was valued at USD 1.5 billion in 2023 and is projected to reach a market size of USD 3.38 billion by 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 12.30%.
Currently, endometrial cancer cannot be diagnosed using progesterone testing. In general, endometrial cancer screening is not advised because many cases are detected early thanks to detectable symptoms. Progesterone levels, however, are connected to endometrial cancer in two different ways. First off, the risk of endometrial cancer is increased by hormonal imbalances, notably high levels of oestrogen and low levels of progesterone. Second, for women who wish to preserve their fertility, progesterone therapy alone may be a therapeutic option for certain endometrial malignancies.
Key Market Insights:
The market for endometrial cancer is moving towards more individualised treatment plans and earlier diagnosis. This presents an opportunity for progesterone testing if it is useful in determining risk or modifying treatment regimens.
The goal of minimally invasive techniques is to ensure patient comfort. The creation of non-invasive, affordable, and dependable progesterone testing for urine or saliva might be a significant trend in the future.
In a perfect world, progesterone testing would enhance current methods for endometrial cancer diagnosis and therapy. This may entail using progesterone levels in conjunction with other risk variables or applying them to customised treatment regimens.
Because of its well-established infrastructure, North America may be an early user of progesterone testing; but, if it turns out to be both affordable and compliant with local healthcare legislation, the Asia-Pacific area, home to a fast-expanding patient population, may see substantial future usage of this test.
Global Progesterone Test for Endometrial Cancer Market Drivers:
Technological Progress Changing the Diagnosis and Treatment of Endometrial Cancer
Technology is changing the landscape of endometrial cancer detection and therapy very quickly, which is good news for patients. Techniques for minimally invasive surgery are one important area of advancement. With smaller incisions, faster recovery periods, and less discomfort following surgery than with open procedures, laparoscopic and robotic operations are becoming more and more popular. Patients will benefit from a more comfortable experience and a quicker return to normal activities as a result. Personalised healthcare is yet another innovative advancement. By using cutting-edge molecular and genetic testing, medical professionals can learn more about the precise mutations causing cancer in their patients. This makes it possible to create individualised treatment programmes with focused medications that target the weaknesses of cancer. When compared to conventional chemotherapy, these tailored medicines frequently have fewer side effects, which enhances the quality of life for patients receiving treatment.
Patient Empowerment: Greater Awareness Promotes Early Detection and Better Results
Educating women about endometrial cancer has proven to be an effective tool in the fight against the illness. Awareness of endometrial cancer risk factors and symptoms is significantly increasing because of lobbying and educational programmes. More women are seeking screening and early diagnosis because of their newly acquired knowledge, which is essential for effective treatment. Early identification enhances patient outcomes and quality of life by enabling minimally invasive treatments and less aggressive treatment alternatives. Women who are more informed are more equipped to speak out for their health and consult with doctors about any troubling symptoms. The prognosis for many women will be greatly improved in the future when endometrial cancer can be identified and treated early thanks to this two-pronged strategy of education and self-advocacy.
Global Progesterone Test for Endometrial Cancer Market Restraints and Challenges:
The Progesterone Test for Endometrial Cancer Market is expected to develop, but there are a few obstacles in its way. The absence of a reliable biomarker for a disease might make diagnosis more difficult and result in pointless biopsies. Progesterone levels are considered, but they don't provide enough detail by themselves. Future research may uncover additional targeted biomarkers, which might alter the function of progesterone testing. Modern diagnostic equipment and therapies are expensive, which strains healthcare resources and restricts access in some areas. Even while these developments are positive, issues of cost and fair access still exist. Limited access to screening programmes can result in delayed diagnosis and worse outcomes, especially in low- and middle-income countries. Enhancing the availability of screening programmes is essential for improving endometrial cancer treatment in its entirety. It is possible to over-diagnose and overtreat, particularly in cases with slow-growing tumours.
Global Progesterone Test for Endometrial Cancer Market Opportunities:
Future potentials are not eliminated by the fact that progesterone testing is not yet available on a specialised market for the detection of endometrial cancer. The potential of progesterone rests in its combination with other agents. First, progesterone levels might start to play a role in risk assessment. Physicians might customise screening recommendations by including progesterone levels in a more comprehensive risk assessment. For example, screening more frequently could be beneficial for women whose progesterone levels are regularly low. Second, progesterone itself is useful as a treatment for some types of endometrial cancer. Treatments with fewer side effects may result from research into novel formulations, delivery systems, and even combinations with other medications. The opportunity essentially resides in creating progesterone-based solutions that enhance current endometrial cancer diagnostic and therapy approaches.
PROGESTERONE TEST FOR ENDOMETRIAL CANCER MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
12.30% |
|
Segments Covered |
By Product type, Application, End User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Abbott Laboratories, Estrellas Life Sciences, Pfizer Inc., Roche Diagnostics, Siemens Healthineers, Teva Pharmaceuticals Industries |
Global Progesterone Test for Endometrial Cancer Market Segmentation: By Product Type
-
Blood Tests
-
Saliva or Urine Tests
Because blood tests are already well-established and well-known, they would be the first choice in medical diagnostics. On the other hand, if non-invasive saliva or urine tests are shown to be dependable, affordable, and easy, providing a more comfortable testing experience for patients, they may emerge as the fastest-growing market.
Global Progesterone Test for Endometrial Cancer Market Segmentation: By Application
-
Risk Stratification
-
Treatment Guidance
Because it focuses on preventive measures, risk stratification which utilises progesterone levels to customise screening recommendations for various patients would probably be the most popular use at first. Treatment guidance, on the other hand, has the potential to develop at the quickest rate if it results in more individualised and successful treatment programmes. Treatment guidance is where progesterone levels dictate treatment options for identified instances.
Global Progesterone Test for Endometrial Cancer Market Segmentation: By End User
-
Hospitals & Clinics
-
Home Testing Kits
Hospitals and clinics, with their preexisting infrastructure and skilled personnel, would probably take the lead initially. The fastest-growing market, though, would be home testing kits but only if the tests are extremely precise, easy to use, and come with clear instructions for what to do afterwards. Additionally, important factors influencing this segment's growth would be regulatory approval and patient comfort with at-home testing.
Global Progesterone Test for Endometrial Cancer Market Segmentation: By Region
-
North America
-
Asia-Pacific
-
Europe
-
South America
-
Middle East and Africa
Progesterone testing may have been adopted early in North America, which is presently leading the way because of established screening and significant healthcare spending. If progesterone testing is easy to use and reasonably priced, it may potentially find considerable application in the Asia-Pacific area, where the patient population and risk factors are developing at a rapid pace. This is only a hypothetical situation, though, based on things like local healthcare laws and the test's unique value offer.
COVID-19 Impact Analysis on the Global Progesterone Test for Endometrial Cancer Market:
The market for endometrial cancer and, thus, any prospective need for progesterone testing in this domain in the future are likely to be indirectly impacted by the COVID-19 pandemic. There may have been delays in screens and diagnosis during the pandemic's peak due to overstretched healthcare resources, which might result in a future spike in demand for diagnostic equipment and therapies to clear this backlog. If progesterone testing becomes a more common risk assessment technique and integrates with remote patient monitoring for endometrial cancer, the pandemic's drive for virtual treatment may also have some bearing. However, COVID-19 limitations may also have impeded research and development, which might have delayed the creation of new diagnostic instruments or progesterone-based treatments for endometrial cancer.
Recent Trends and Developments in the Global Progesterone Test for Endometrial Cancer Market:
The Progesterone Test for Endometrial Cancer Market is changing in favour of individualised treatment plans and early diagnosis. This offers fascinating prospects for future progesterone testing. The emphasis on early diagnosis is a significant trend that is driving research into better screening techniques. A non-invasive progesterone test for risk assessment could out to be a useful instrument. Furthermore, the emergence of personalised medicine emphasises the necessity of testing to guide customised treatment regimens. Progesterone tests might be included in this strategy if progesterone levels show a greater correlation with endometrial cancer subtypes. Moreover, diagnostics is another area where minimally invasive methods are highlighted. Another trend for the future might be the creation of accurate and reasonably priced non-invasive progesterone testing, possibly utilising urine or saliva samples.
Key Players:
-
Abbott Laboratories
-
Estrellas Life Sciences
-
Pfizer Inc.
-
Roche Diagnostics
-
Siemens Healthineers
-
Teva Pharmaceuticals Industries
Chapter 1. Progesterone Test for Endometrial Cancer Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Progesterone Test for Endometrial Cancer Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Progesterone Test for Endometrial Cancer Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Progesterone Test for Endometrial Cancer Market - Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Progesterone Test for Endometrial Cancer Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Progesterone Test for Endometrial Cancer Market – By Product Type
6.1 Introduction/Key Findings
6.2 Blood Tests
6.3 Saliva or Urine Tests
6.4 Y-O-Y Growth trend Analysis By Product Type
6.5 Absolute $ Opportunity Analysis By Product Type, 2024-2030
Chapter 7. Progesterone Test for Endometrial Cancer Market – By Application
7.1 Introduction/Key Findings
7.2 Risk Stratification
7.3 Treatment Guidance
7.4 Y-O-Y Growth trend Analysis By Application
7.5 Absolute $ Opportunity Analysis By Application, 2024-2030
Chapter 8. Progesterone Test for Endometrial Cancer Market – By End User
8.1 Introduction/Key Findings
8.2 Hospitals & Clinics
8.3 Home Testing Kits
8.4 Y-O-Y Growth trend Analysis By End User
8.5 Absolute $ Opportunity Analysis By End User, 2024-2030
Chapter 9. Progesterone Test for Endometrial Cancer Market , By Geography – Market Size, Forecast, Trends & Insights
9.1 North America
9.1.1 By Country
9.1.1.1 U.S.A.
9.1.1.2 Canada
9.1.1.3 Mexico
9.1.2 By Product Type
9.1.3 By Application
9.1.4 By End User
9.1.5 Countries & Segments - Market Attractiveness Analysis
9.2 Europe
9.2.1 By Country
9.2.1.1 U.K
9.2.1.2 Germany
9.2.1.3 France
9.2.1.4 Italy
9.2.1.5 Spain
9.2.1.6 Rest of Europe
9.2.2 By Product Type
9.2.3 By Application
9.2.4 By End User
9.2.5 Countries & Segments - Market Attractiveness Analysis
9.3 Asia Pacific
9.3.1 By Country
9.3.1.1 China
9.3.1.2 Japan
9.3.1.3 South Korea
9.3.1.4 India
9.3.1.5 Australia & New Zealand
9.3.1.6 Rest of Asia-Pacific
9.3.2 By Product Type
9.3.3 By Application
9.3.4 By End User
9.3.5 Countries & Segments - Market Attractiveness Analysis
9.4 South America
9.4.1 By Country
9.4.1.1 Brazil
9.4.1.2 Argentina
9.4.1.3 Colombia
9.4.1.4 Chile
9.4.1.5 Rest of South America
9.4.2 By Product Type
9.4.3 By Application
9.4.4 By Application
9.4.5 Countries & Segments - Market Attractiveness Analysis
9.5 Middle East & Africa
9.5.1 By Country
9.5.1.1 United Arab Emirates (UAE)
9.5.1.2 Saudi Arabia
9.5.1.3 Qatar
9.5.1.4 Israel
9.5.1.5 South Africa
9.5.1.6 Nigeria
9.5.1.7 Kenya
9.5.1.8 Egypt
9.5.1.9 Rest of MEA
9.5.2 By Product Type
9.5.3 By Application
9.5.4 By End User
9.5.5 Countries & Segments - Market Attractiveness Analysis
Chapter 10. Progesterone Test for Endometrial Cancer Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
10.1 Abbott Laboratories
10.2 Estrellas Life Sciences
10.3 Pfizer Inc.
10.4 Roche Diagnostics
10.5 Siemens Healthineers
10.6 Teva Pharmaceuticals Industries
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The Global Progesterone Test for Endometrial Cancer Market size is valued at USD 1.5 billion in 2023.
The worldwide Global Progesterone Test for Endometrial Cancer Market growth is estimated to be 12.30% from 2024 to 2030.
The Global Progesterone Test for Endometrial Cancer Market segmentation covered in the report are By Product Type (Blood Tests, Saliva or Urine Tests); By Application (Risk Stratification, Treatment Guidance); By End User (Hospitals & Clinics, Home Testing Kits) and by region.
The following might impact the market for progesterone tests for endometrial cancer in the future: Progesterone testing without invasive procedures for risk assessment Combining personalised medicine with endometrial cancer therapy integration.
The global progesterone test for endometrial cancer market is probably indirectly affected by the COVID-19 pandemic. The pandemic's effects on screening and diagnosis might increase demand for diagnostic instruments in the future, possibly incorporating progesterone-based assays. However, the development of this application may be slowed down by COVID-19-related research delays.