Preeclampsia Diagnostics Market Size (2024 – 2030)
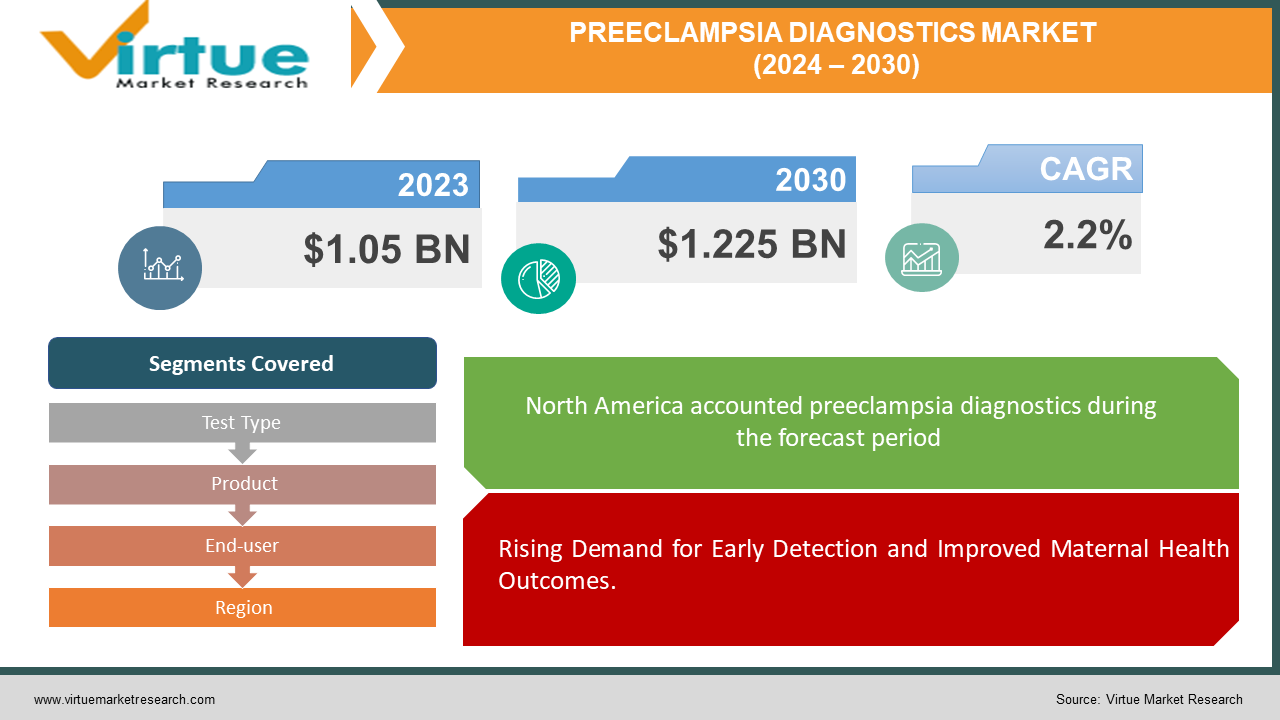
The Global Preeclampsia Diagnostics Market was valued at USD 1.05 billion and is projected to reach a market size of USD 1.225 billion by the end of 2030. The market is anticipated to expand at a compound annual growth rate (CAGR) of 2.2% between 2024 and 2030.
Preeclampsia, often called toxaemia, is a pregnancy problem that progresses quickly and is characterized by high blood pressure, proteinuria, hypertension, excessive protein in the mother's urine, and organ damage, usually to the kidneys and liver. Serious outcomes for both the woman and the child may arise from it, such as low birth weight, early delivery, and in extreme situations, maternal and fetal death. Preeclampsia is diagnosed using a variety of techniques, usually in combination. To diagnose the illness, gauge its severity, and keep an eye on the mother's and baby's health, many tests and procedures are involved. Blood pressure monitoring, urine and blood tests to measure specific biomarkers, and ultrasound imaging to assess baby growth and well-being are a few of the often-used diagnostic techniques. They are carried out to identify and treat preeclampsia and lower the mother's and infant's risk of problems. Preeclampsia diagnostic techniques are extensively used in hospitals, assisted living facilities, and diagnostic centers because of these advantages.
Key Market Insights:
Preeclampsia affects 5-8% of pregnancies globally, necessitating improved diagnostics for early detection to enhance maternal and fetal outcomes.
Non-invasive blood tests for preeclampsia, boasting over 90% accuracy rates, are gaining traction as a convenient alternative to traditional methods. Artificial intelligence (AI) integration in preeclampsia diagnostics enhances prediction accuracy by analyzing data from various sources like blood tests and ultrasound scans.
The global preeclampsia diagnostics market saw a 7.8% growth in 2023 compared to 2022.There's a rising emphasis on developing affordable diagnostic tools for preeclampsia, particularly in low-resource settings, aiming to improve healthcare outcomes globally.
The global preeclampsia diagnostics market is expected to grow over 7.5% annually, emphasizing the increasing focus on early diagnosis to mitigate complications.
Preeclampsia Diagnostics Market Drivers:
Rising Demand for Early Detection and Improved Maternal Health Outcomes.
The global preeclampsia diagnostics market is experiencing a surge driven by the increasing emphasis on early detection and improved maternal health outcomes. Preeclampsia, a serious pregnancy complication characterized by high blood pressure and potential organ damage, can pose significant risks to both mother and baby. Early diagnosis is crucial for timely intervention and management, preventing complications like preterm birth, eclampsia (seizures), and even death. This growing awareness has fueled a demand for accurate and reliable diagnostic methods. Traditional methods often rely on monitoring blood pressure and urine protein levels, which may not always be definitive or provide early enough warning signs. Advanced diagnostic tools, particularly biomarker-based tests, offer a more precise and potentially earlier detection of preeclampsia. These tests measure specific biological markers in the mother's blood or placenta that are associated with an increased risk of developing the condition. By identifying women at risk in the early stages of pregnancy, healthcare providers can implement preventive measures and closely monitor their health, significantly improving pregnancy outcomes for both mothers and babies. This focus on early detection aligns with broader trends in women's healthcare, where preventative measures and early intervention are increasingly prioritized. As the understanding of preeclampsia and its potential complications grows, the demand for effective diagnostic tools is expected to continue rising, propelling the preeclampsia diagnostics market forward.
Technological Advancements and Development of Novel Diagnostic Techniques.
Another key driver of the global preeclampsia diagnostics market is the continuous development of novel diagnostic techniques. Research and development efforts are directed towards creating more efficient, non-invasive, and highly accurate methods for identifying preeclampsia. Biomarker-based tests, as mentioned earlier, represent a significant advancement, offering a more objective and potentially earlier detection compared to traditional methods. Beyond blood tests, researchers are exploring the potential of other diagnostic tools. Imaging techniques like Doppler ultrasound are being investigated for their ability to detect preeclampsia by examining blood flow patterns in the placenta. Additionally, there's ongoing research into genetic markers that may be linked to an increased risk of developing preeclampsia. These advancements hold promise for creating a comprehensive diagnostic approach, allowing healthcare professionals to identify preeclampsia with greater accuracy and efficiency. The ongoing development of novel diagnostic techniques is not only driven by the need for improved accuracy but also by a desire for less invasive and more patient-friendly procedures. Early detection often involves multiple doctor visits and blood tests, which can be burdensome for pregnant women. New technologies that are less invasive or provide quicker results could significantly improve the patient experience and encourage women to seek prenatal care more readily. As research in preeclampsia diagnostics continues to evolve, we can expect to see the emergence of even more sophisticated and user-friendly tools, further propelling the growth of this market.
Global Preeclampsia Diagnostics Market Restraints and Challenges:
Preeclampsia diagnostics have made significant strides, however, the market is still limited and confronted with difficulties. The shortcomings of the diagnostic instruments available today represent a major obstacle. Compared to conventional techniques, biomarker-based testing allows for earlier detection, but they are not always conclusive. False positives and negatives can happen, which could cause needless worry or postpone important treatments. Furthermore, some advanced tests may not be as accessible due to their high cost, especially in low- and middle-income nations. Moreover, the absence of uniform guidelines for diagnosing preeclampsia in various healthcare environments might lead to discrepancies and impede the extensive integration of novel technologies. Furthermore, new diagnostic instruments may take longer to reach the market due to regulatory obstacles. Long and stringent licensing procedures for novel tests have the potential to stifle innovation and restrict patient access to the most recent developments. Lastly, a problem that may arise in resource-constrained environments is the lack of trained healthcare personnel who are proficient in interpreting and applying these novel diagnostic instruments. For the preeclampsia diagnostics market to attain its full potential and guarantee improved maternal health outcomes globally, these constraints and hurdles must be addressed by ongoing research, increased standardization, and improved training for healthcare professionals.
Global Preeclampsia Diagnostics Market Opportunities:
The global preeclampsia diagnostics market is experiencing a surge in opportunities, driven by technological advancements and evolving healthcare demands. A pivotal area of focus is the development of combination diagnostic approaches, which integrate biomarker testing, imaging techniques, and genetic analysis to offer a more comprehensive assessment of preeclampsia risk. This multi-faceted approach holds the potential to enhance diagnostic accuracy, enabling earlier detection and better risk stratification, ultimately leading to improved pregnancy outcomes. Moreover, the emphasis on personalized medicine further propels market growth, as tailored approaches based on individual risk profiles can optimize patient care and intervention strategies. The prospect of non-invasive prenatal tests (NIPTs) dedicated to preeclampsia detection presents another promising avenue, offering simplicity, patient compliance, and enhanced early detection rates through a simple blood draw. With ongoing research and development efforts aimed at overcoming challenges and exploring novel solutions, the future of the preeclampsia diagnostics market is poised for significant expansion. These advancements are poised to revolutionize preeclampsia diagnosis, ensuring safer pregnancies and healthier outcomes for mothers and babies worldwide.
PREECLAMPSIA DIAGNOSTICS MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
2.2% |
|
Segments Covered |
By Test Type, Product, End-user, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Diabetomics Inc., DRG Instruments GmbH, F. Hoffmann-La Roche Ltd., Metabolomic Diagnostics Limited, MOMM Diagnostics, PerkinElmer Inc., Sera Prognostics, Siemens Healthineers AG, Thermo Fisher Scientific Inc. |
Global Preeclampsia Diagnostics Market Segmentation: By Test Type
-
Blood Tests
-
Urine Analysis
The Global Preeclampsia Diagnostics Market is Segmented by Test Type, Blood Tests held the largest market share last year and is poised to maintain its dominance throughout the forecast period. Preeclampsia diagnostics market growth is expected to be particularly strong in the blood tests segment due to rising incidence rates and improved biomarker detection. The need for screening tests is being further increased by the growing adoption of blood testing by healthcare facilities for the identification of toxaemia. To detect preeclampsia, for instance, the National Institute for Health and Care Excellence (NICE) advised certain blood tests. Urine analysis, on the other hand, is expected to expand significantly because it is the preferred method for diagnosing diseases early on, especially in emerging nations. Urine analysis has the potential to increase in a particular market niche because of its accessibility, convenience, and non-invasive nature. These developments in diagnostic methods could improve preeclampsia early diagnosis and therapy, leading to better outcomes for the health of the mother and fetus.
Global Preeclampsia Diagnostics Market Segmentation: By Product
-
Instruments
-
Consumables
The Global Preeclampsia Diagnostics Market is Segmented by Product, Instruments held the largest market share last year and is poised to maintain its dominance throughout the forecast period. In the preeclampsia diagnostics industry, the consumables category emerged as the top market share holder and is expected to maintain the highest Compound Annual Growth Rate (CAGR) throughout the projection period. The prevalence of biomarker-based diagnosis techniques and the rise in the number of pregnant women suffering from preeclampsia are the main drivers of this growth trajectory. Further driving the need for consumables shortly is expected to be increased awareness of preeclampsia and the tools used to identify it. The International Federation of Gynaecology and Obstetrics (FIGO) is expected to promote segmental growth by raising gynecologists' awareness of available reagents and kits through the issuance of new recommendations that advocate biomarker testing methods. In the meanwhile, the market for instruments is expected to grow rapidly in the years to come due to a rise in the number of people with hypertensive pregnancy problems, which increase the risk of toxaemia. In addition, the growing need for centers that diagnose urological diseases is anticipated to fuel the segment's growth in the years to come. These advancements highlight how crucial consumables and equipment are to improving maternal healthcare outcomes worldwide and preeclampsia detection advancement.
Global Preeclampsia Diagnostics Market Segmentation: By End-user
-
Hospitals
-
Specialty Clinics
-
Diagnostic Centers
The Global Preeclampsia Diagnostics Market is Segmented by End-user, Hospitals held the largest market share last year and are poised to maintain their dominance throughout the forecast period. During the forecast period, the hospitals sector, which currently has the second-largest market share, is expected to expand at a moderate Compound Annual Growth Rate (CAGR). A growing number of in-hospital visits from patients suffering from toxaemia is the main cause of this growth. Additionally, increased hospitalization rates, improved healthcare infrastructures, and the rise of hospitals in developing nations are anticipated to support segmental growth. The diagnosis centers category is anticipated to grow at the fastest rate throughout the same time frame. This increase is linked to an increase in the number of pregnant patients with preeclampsia visiting diagnostic facilities. Additionally, these centers place a high priority on offering enhanced screening for preeclampsia during pregnancy, both late-onset and early-onset, as this is expected to propel segment expansion. When taken as a whole, these developments demonstrate the critical role that hospitals and diagnostic centers play in meeting the diagnostic requirements of people who have preeclampsia and so making a major global contribution to improving maternal healthcare outcomes.
Global Preeclampsia Diagnostics Market Segmentation: By Region
-
North America
-
Asia-Pacific
-
Europe
-
South America
-
Middle East and Africa
The Global Preeclampsia Diagnostics Market is Segmented by Region, North America held the largest market share last year and is poised to maintain its dominance throughout the forecast period. This region's market is growing because of the high uptake of technologically improved products, growing patient and provider awareness of the seriousness of the disease, and substantial healthcare spending. Furthermore, the market is anticipated to rise as more cutting-edge preeclampsia diagnostics products are introduced. The preeclampsia diagnostics market share was led by Europe, which is expected to grow significantly over the projection period. The increase can be attributed to the rising number of patients with pregnancy-related hypertension problems. Furthermore, a rise in government healthcare organizations' approvals of the diagnosis of hypertensive problems during pregnancy could encourage market expansion in Europe. The Asia-Pacific region is experiencing some issues, including a high pregnancy rate, rising disease awareness, and an increase in the use of reproductive treatments. The region's greatest CAGR can be attributed to the rising number of expectant mothers. Throughout the projected period, Latin America the Middle East, and Africa are anticipated to grow at a relatively slower CAGR. The increase can be attributed to both the rising cost of healthcare and the growing prevalence of toxaemia.
COVID-19 Impact Analysis on the Global Preeclampsia Diagnostics Market:
The COVID-19 pandemic brought about a mixed impact on the global preeclampsia diagnostics market. Initially disrupting healthcare services and potentially delaying prenatal care, it also emphasized the importance of early detection and close monitoring of high-risk pregnancies. Heightened awareness of the increased risk of preeclampsia among pregnant women with COVID-19 may have led to more diagnoses as healthcare providers intensified monitoring efforts. However, challenges emerged as resource allocation shifted towards pandemic-related research and treatment, potentially delaying clinical trials and approvals for new diagnostic tools. Lockdowns and movement restrictions may have further limited access to prenatal care and diagnostics, particularly in regions with weaker healthcare infrastructure. Overall, while the pandemic may have temporarily boosted preeclampsia diagnoses, disruptions to healthcare services and delayed approvals for new technologies likely counterbalanced this effect. Looking ahead, the pandemic's focus on maternal health could catalyze increased investment and innovation in preeclampsia diagnostics in the long term.
Latest Trends/ Developments:
Preeclampsia diagnostics is a global sector booming with exciting new developments. The investigation of machine learning and artificial intelligence (AI) for preeclampsia prediction is a major trend. Artificial intelligence (AI) can detect minute patterns and risk factors that conventional approaches could overlook by scrutinizing extensive datasets containing patient medical records, demographics, and biomarker levels. This might result in more precise risk categorization, which would enable medical professionals to prioritize interventions for women who are most at risk and tailor care programs specifically for them. The creation of point-of-care (POC) diagnostic testing is another emerging trend. Preeclampsia screening could be done at home during prenatal visits or at the doctor's office with the help of these portable and easy-to-use assays. POC tests have the potential to greatly increase accessibility, especially in low-resource or distant locations where access to specialized diagnostic facilities may be restricted. Furthermore, scientists are still investigating the possibility of placental proteins and exosomes, which are tiny membrane vesicles, serving as early signs of preeclampsia in their ongoing research into novel biomarkers. These developments could lead to the development of a more complete diagnostic toolkit for preeclampsia, which could lead to earlier identification, better pregnancy outcomes, and eventually, better health outcomes for moms and their offspring.
Key players:
-
Diabetomics Inc.
-
DRG Instruments GmbH
-
F. Hoffmann-La Roche Ltd.
-
Metabolomic Diagnostics Limited
-
MOMM Diagnostics
-
PerkinElmer Inc.
-
Sera Prognostics
-
Siemens Healthineers AG
-
Thermo Fisher Scientific Inc.
Chapter 1. Preeclampsia Diagnostics Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Preeclampsia Diagnostics Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Preeclampsia Diagnostics Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Preeclampsia Diagnostics Market Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Preeclampsia Diagnostics Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Preeclampsia Diagnostics Market – By Test Type
6.1 Introduction/Key Findings
6.2 Blood Tests
6.3 Urine Analysis
6.4 Y-O-Y Growth trend Analysis By Test Type
6.5 Absolute $ Opportunity Analysis By Test Type, 2024-2030
Chapter 7. Preeclampsia Diagnostics Market – By Product
7.1 Introduction/Key Findings
7.2 Instruments
7.3 Consumables
7.4 Y-O-Y Growth trend Analysis By Product
7.5 Absolute $ Opportunity Analysis By Product, 2024-2030
Chapter 8. Preeclampsia Diagnostics Market – By End-user
8.1 Introduction/Key Findings
8.2 Hospitals
8.3 Specialty Clinics
8.4 Diagnostic Centers
8.5 Y-O-Y Growth trend Analysis By End-user
8.6 Absolute $ Opportunity Analysis By End-user, 2024-2030
Chapter 9. Preeclampsia Diagnostics Market , By Geography – Market Size, Forecast, Trends & Insights
9.1 North America
9.1.1 By Country
9.1.1.1 U.S.A.
9.1.1.2 Canada
9.1.1.3 Mexico
9.1.2 By Test Type
9.1.3 By Product
9.1.4 By End-user
9.1.5 Countries & Segments - Market Attractiveness Analysis
9.2 Europe
9.2.1 By Country
9.2.1.1 U.K
9.2.1.2 Germany
9.2.1.3 France
9.2.1.4 Italy
9.2.1.5 Spain
9.2.1.6 Rest of Europe
9.2.2 By Test Type
9.2.3 By Product
9.2.4 By End-user
9.2.5 Countries & Segments - Market Attractiveness Analysis
9.3 Asia Pacific
9.3.1 By Country
9.3.1.1 China
9.3.1.2 Japan
9.3.1.3 South Korea
9.3.1.4 India
9.3.1.5 Australia & New Zealand
9.3.1.6 Rest of Asia-Pacific
9.3.2 By Test Type
9.3.3 By Product
9.3.4 By End-user
9.3.5 Countries & Segments - Market Attractiveness Analysis
9.4 South America
9.4.1 By Country
9.4.1.1 Brazil
9.4.1.2 Argentina
9.4.1.3 Colombia
9.4.1.4 Chile
9.4.1.5 Rest of South America
9.4.2 By Test Type
9.4.3 By Product
9.4.4 By End-user
9.4.5 Countries & Segments - Market Attractiveness Analysis
9.5 Middle East & Africa
9.5.1 By Country
9.5.1.1 United Arab Emirates (UAE)
9.5.1.2 Saudi Arabia
9.5.1.3 Qatar
9.5.1.4 Israel
9.5.1.5 South Africa
9.5.1.6 Nigeria
9.5.1.7 Kenya
9.5.1.8 Egypt
9.5.1.9 Rest of MEA
9.5.2 By Test Type
9.5.3 By Product
9.5.4 By End-user
9.5.5 Countries & Segments - Market Attractiveness Analysis
Chapter 10. Preeclampsia Diagnostics Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
10.1 Diabetomics Inc.
10.2 DRG Instruments GmbH
10.3 F. Hoffmann-La Roche Ltd.
10.4 Metabolomic Diagnostics Limited
10.5 MOMM Diagnostics
10.6 PerkinElmer Inc.
10.7 Sera Prognostics
10.8 Siemens Healthineers AG
10.9 Thermo Fisher Scientific Inc.
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
By 2023, the Preeclampsia Diagnostics market is expected to be valued at USD 1.05 billion.
Through 2030, the global Preeclampsia Diagnostics market is expected to grow at a CAGR of 2.2%.
By 2030, the global Preeclampsia Diagnostics market is expected to grow to a value of USD 1.225 billion.
North America is predicted to lead the market for global Preeclampsia Diagnostics.
The global Preeclampsia Diagnostics market has segments of Test Type, Product, End Use, And Region.



