Point of Care Testing for Systemic Lupus Erythematosus (SLE) Market Size (2023-2030)
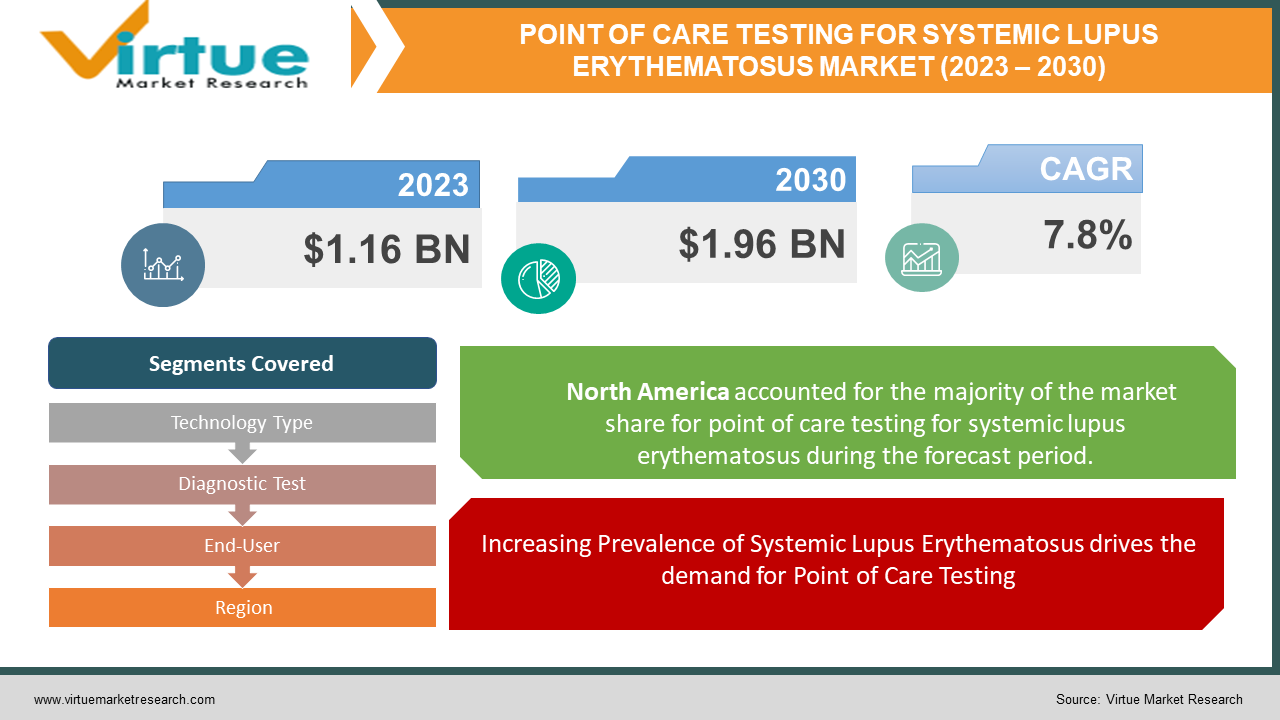
The Global Point of care testing for SLE market was valued at USD 1.16 billion and is projected to reach a market size of USD 1.96 billion by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 7.8%.
Systemic Lupus Erythematosus (SLE) is a complex autoimmune disease affecting multiple organs, making timely and accurate diagnosis crucial for effective management and improved patient outcomes. Point of Care Testing (POCT) for SLE has emerged as a vital diagnostic tool, enabling rapid and convenient testing directly at the patient's location. The market for POCT in SLE is witnessing remarkable growth due to the rising prevalence of the disease and the necessity of early detection to prevent disease progression. Healthcare providers are increasingly adopting POCT for SLE due to its advantages, including quick results, reduced turnaround time, and enhanced accessibility, which further fuels its expansion. As this diagnostic approach continues to evolve, its impact on early diagnosis and patient care is expected to be profound, revolutionizing the management of SLE and improving the lives of affected individuals.
Global Point of Care Testing for Systemic Lupus Erythematosus (SLE) Market Drivers:
Increasing Prevalence of Systemic Lupus Erythematosus drives the demand for Point of Care Testing.
Systemic Lupus Erythematosus (SLE) is a chronic autoimmune disease that is becoming increasingly prevalent worldwide. This rise in incidence highlights the need for effective and easily accessible diagnostic solutions to identify the disease at its early stages. Point of Care Testing (POCT) for SLE has emerged as a valuable tool, providing rapid and accurate results directly at the patient's location, and facilitating timely intervention and personalized treatment plans. Healthcare providers are prioritizing early diagnosis and improved disease management, leading to a significant surge in the demand for POCT for SLE.
Advantages of Point of Care Testing fuels market expansion.
The market expansion of Point of Care Testing (POCT) for Systemic Lupus Erythematosus (SLE) can be attributed to several advantages it offers. These include the rapid availability of test results, reduced turnaround time, portability, and accessibility in various healthcare settings. With POCT, healthcare providers can make quick decisions, leading to early detection and timely treatment initiation for SLE patients. By enabling tests to be conducted directly at the patient's location, POCT alleviates the burden on centralized laboratories and streamlines the diagnostic process, making it an invaluable tool in modern healthcare practices.
Growing Awareness of Early Disease Detection fosters demand for POCT for SLE.
Timely detection of Systemic Lupus Erythematosus is of paramount importance in effectively managing the disease and enhancing patient outcomes. Point of Care Testing (POCT) for SLE offers a promising approach to achieve early disease detection, facilitating prompt intervention and personalized therapies. The growing awareness among healthcare professionals and patients regarding the advantages of early diagnosis is a key driver behind the increasing demand for POCT for SLE. As stakeholders in the healthcare industry increasingly recognize the significance of early disease management, the adoption of Point of Care Testing for SLE is expected to witness substantial growth.
Global Point of Care Testing for Systemic Lupus Erythematosus (SLE) Market Challenges:
The complexity of SLE Diagnosis poses challenges to Point of Care Testing.
Systemic Lupus Erythematosus (SLE) is a complex autoimmune disease known for its varied clinical presentations, making its diagnosis a challenge. Point of Care Testing (POCT) for SLE must effectively address these intricacies to ensure the utmost accuracy and reliability. Healthcare providers and diagnostic manufacturers are confronted with the task of developing POCT solutions that encompass the diverse spectrum of SLE manifestations and provide comprehensive diagnostic insights. Successfully overcoming these diagnostic complexities and ensuring the precision of POCT for SLE are crucial steps in achieving widespread adoption of this valuable diagnostic tool.
Regulatory Compliance and Quality Assurance present barriers to market entry.
The development and commercialization of Point of Care Testing (POCT) for Systemic Lupus Erythematosus (SLE) necessitate strict compliance with rigorous regulatory standards and quality assurance measures. Diagnostic manufacturers must undergo a thorough process of obtaining regulatory approvals and ensuring adherence to stringent quality control procedures. This endeavor can be time-consuming and resource-intensive, but it is crucial to guarantee the safety and effectiveness of POCT for SLE. Market players in this field must make significant investments in robust quality assurance processes and proactively engage with regulatory authorities early in the development phase to overcome these barriers and successfully penetrate the market.
Global Point of Care Testing for Systemic Lupus Erythematosus (SLE) Market Opportunities:
Technological Advancements drive innovations in POCT for SLE.
Continuous research and development efforts in the field of Point of Care Testing (POCT) for Systemic Lupus Erythematosus (SLE) offer exciting opportunities for technological advancements and innovation. Industry players can capitalize on these opportunities by investing in research collaborations and exploring cutting-edge technologies. Through such endeavors, the development of next-generation POCT solutions for SLE is possible, featuring improved sensitivity, specificity, and user-friendliness. These advancements will contribute to early and accurate diagnosis, ultimately enhancing patient care. Embracing innovation in POCT for SLE will drive its evolution, making it more efficient and effective in modern healthcare practices.
Growing Emphasis on Personalized Medicine enhances market prospects.
Personalized medicine is gaining momentum in the healthcare industry, and Point of Care Testing (POCT) for Systemic Lupus Erythematosus (SLE) can play a crucial role in this context. The concept of tailoring diagnostic and treatment approaches to individual patients based on their unique disease manifestations and responses to therapies is a central focus in modern medicine. POCT for SLE, with its ability to provide rapid and targeted diagnostic insights, aligns perfectly with the principles of personalized medicine. This diagnostic tool can aid healthcare professionals in efficiently assessing each patient's condition, leading to more tailored and effective treatment plans. By capitalizing on the growing emphasis on personalized care, diagnostic manufacturers have the opportunity to expand the market reach of Point of Care Testing for SLE.
POINT OF CARE TESTING FOR SYSTEMIC LUPUS ERYTHEMATOSUS MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2022 - 2030 |
|
Base Year |
2022 |
|
Forecast Period |
2023 - 2030 |
|
CAGR |
7.8% |
|
Segments Covered |
By Technology Type, Diagnostic Test, End-User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Perfern RF, Rapid Labs, Arlington Scientific, Avantor, Atlas Medical |
Point of Care Testing for SLE Market Segmentation – By Technology Type
-
Immunofluorescence Assays (IFA)
-
Enzyme-Linked Immunosorbent Assays (ELISA)
-
Molecular Diagnostics (PCR-based)
Based on the segmentation by Technology Type, Enzyme-Linked Immunosorbent Assay (ELISA) is the most used. It is widely utilized in various diagnostic settings due to its high sensitivity, specificity, and ability to detect specific antibodies associated with SLE. It is a well-established and reliable method for detecting autoimmune antibodies in patient samples, making it a popular choice for SLE diagnosis.
However, Immunofluorescence assay (IFA) is second in order. IFA involves using specific antibodies labeled with fluorescent dyes to detect autoantibodies in patient samples. It is known for its sensitivity and ability to detect specific antibodies related to SLE, making it a valuable tool in the diagnostic process.
Global Point of Care Testing for Systemic Lupus Erythematosus (SLE) Market Segmentation: By Diagnostic Test
-
Antinuclear Antibody (ANA) Test
-
Autoantibody Test
-
Compliment Component Test
-
Other Tests
Based on market segmentation by Test Type, the Antinuclear Antibody (ANA) Test currently holds the largest share in the Point of Care Testing for the SLE market. The ANA Test is a primary screening tool for SLE, detecting the presence of autoantibodies that are characteristic of the disease. As the demand for early disease detection rises, the adoption of ANA testing at the point of care is expected to increase, contributing to market growth. However, the Autoantibody Test is the second most shareholder in the segment. Autoantibody Test is used to detect specific autoantibodies that are associated with SLE and other autoimmune diseases. These autoantibodies target various components of the body's cells and tissues, and their presence helps in the diagnosis and classification of SLE.
Global Point of Care Testing for Systemic Lupus Erythematosus (SLE) Market Segmentation: By End-User
-
Hospitals
-
Clinics
-
Home Care
-
Other
Based on the segmentation by End-User, both Hospitals currently dominate the Point of Care Testing for the SLE market due to their role as primary healthcare settings where SLE diagnosis and management are conducted. The integration of POCT for SLE in hospitals and clinics enhances the efficiency of diagnostic procedures and enables timely treatment decisions. However, the adoption of POCT for SLE in Homecare Settings is growing steadily with the increasing demand for decentralized testing and patient-centered care.
Global Point of Care Testing for Systemic Lupus Erythematosus (SLE) Market Segmentation: By Region
-
North America
-
Europe
-
Asia-Pacific
-
Middle East and Africa
-
South America
Based on market segmentation by Region, North America currently holds the largest share in the Point of Care Testing for Systemic Lupus Erythematosus (SLE) market, driven by the region's well-established healthcare infrastructure and high awareness about SLE and POCT solutions. However, the Asia-Pacific region is the fastest-growing market for POCT for SLE, fuelled by the rising prevalence of SLE, increasing healthcare expenditure, and the growing adoption of advanced diagnostic technologies in the region.
Recent Industry Developments:
In June 2023, Lupus Therapeutics Partners to Evaluate Potential treatment for SLE and Lupus Nephritis through the North American Trial Network. In February 2023, A team of researchers at the University of Houston reported the success of their new method for the early diagnosis and monitoring of lupus nephritis—at home. The home test—with results read on a smartphone—is meant to eventually replace the gold standard for diagnosis of active LN, an invasive kidney biopsy.
Global Point of Care Testing for Systemic Lupus Erythematosus (SLE) Market Key Players:
-
Perfern RF
-
Rapid Labs
-
Arlington Scientific
-
Avantor
-
Atlas Medical
Chapter 1. Point of Care Testing for SLE Market - Scope & Methodology
1.1 Market Segmentation
1.2 Assumptions
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Point of Care Testing for SLE Market - Executive Summary
2.1 Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.3 COVID-19 Impact Analysis
2.3.1 Impact during 2023 – 2030
2.3.2 Impact on Supply – Demand
Chapter 3. Point of Care Testing for SLE Market - Competition Scenario
3.1 Market Share Analysis
3.2 Product Benchmarking
3.3 Competitive Strategy & Development Scenario
3.4 Competitive Pricing Analysis
3.5 Supplier - Distributor Analysis
Chapter 4. Point of Care Testing for SLE Market - Entry Scenario
4.1 Case Studies – Start-up/Thriving Companies
4.2 Regulatory Scenario - By Region
4.3 Customer Analysis
4.4 Porter's Five Force Model
4.4.1 Bargaining Power of Suppliers
4.4.2 Bargaining Powers of Customers
4.4.3 Threat of New Entrants
4.4.4 Rivalry among Existing Players
4.4.5 Threat of Substitutes
Chapter 5. Point of Care Testing for SLE Market - Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Point of Care Testing for SLE Market - By Technology Type
6.1 Immunofluorescence Assays (IFA)
6.2 Enzyme-Linked Immunosorbent Assays (ELISA)
6.3 Molecular Diagnostics (PCR-based)
Chapter 7. Point of Care Testing for SLE Market - By Diagnostic Test
7.1 Antinuclear Antibody (ANA) Test
7.2 Autoantibody Test
7.3 Compliment Component Test
7.4 Other Tests
Chapter 8. Point of Care Testing for SLE Market - By End-User
8.1 Hospitals
8.2 Clinics
8.3 Home Care
8.4 Other
Chapter 9. Point of Care Testing for SLE Market – By Region
9.1 North America
9.2 Europe
9.3 Asia-Pacific
9.4 Latin America
9.5 The Middle East
9.6 Africa
Chapter 10. Point of Care Testing for SLE Market – Key players
10.1 Perfern RF
10.2 Rapid Labs
10.3 Arlington Scientific
10.4 Avantor
10.5 Atlas Medical
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The Global Point of Care Testing for Systemic Lupus Erythematosus (SLE) Market was valued at USD 1.08 billion in 2022 and is projected to reach USD 1.97 billion by 2030, exhibiting a robust CAGR of 7.8% during the forecast period from 2023 to 2030.
The increasing prevalence of Systemic Lupus Erythematosus, the advantages of Point of Care Testing, and growing awareness of early disease detection are the market drivers for Point of Care Testing for SLE.
The segments under the Point of Care Testing for the SLE market by Test Type include Antinuclear Antibody (ANA) Test, Anti-dsDNA Test, Anti-Smith (Sm) Test, and Other Tests.
The Asia-Pacific region is the fastest growing due to increasing healthcare expenditure, rising awareness of SLE, and the adoption of advanced diagnostic technologies in the region.
North America currently holds the largest share in the Point of Care Testing for Systemic Lupus Erythematosus (SLE) market, driven by the region's well-established healthcare infrastructure and high awareness about SLE and POCT solutions.



