Point of Care Testing for Erythrodermic Psoriasis Market Size (2024 – 2030)
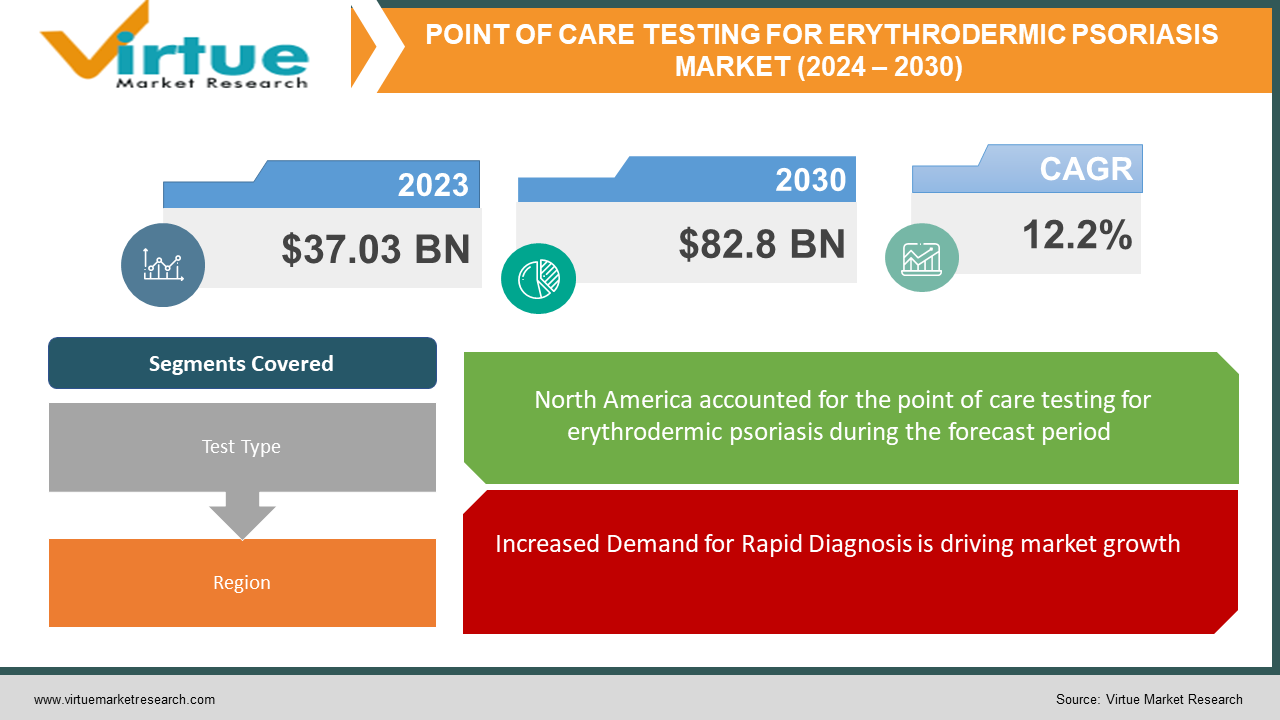
The global point-of-care testing (POCT) market size was estimated at USD 37.03 billion in the year 2023 and is expected to reach around USD 82.8 billion by 2030 with an increasing CAGR of 12.2% from 2023 to 2030 due to increasing demands from the market due to increasing disorders among the population.
Point-of-care testing (POCT) for erythrodermic psoriasis aims to bring rapid and accurate diagnosis directly to the doctor's office or even the patient's bedside. Instead of waiting for lab results, POCT devices use blood or skin samples to detect biomarkers specific to this severe form of psoriasis. This faster diagnosis allows for quicker treatment initiation, potentially improving patient outcomes and reducing the risk of complications. While the market size is currently unclear due to its niche nature, the growing demand for rapid psoriasis diagnosis and the general trend towards POCT suggest potential for future growth in this area.
Key Market Insights:
The Point-of-Care Testing (POCT) market for erythrodermic psoriasis is experiencing a surge, fueled by several key factors. Rising psoriasis prevalence globally creates a strong demand for faster and more accessible diagnostic tools. POCT's advantages shine here, offering rapid results, improved patient convenience, and potentially lower healthcare costs compared to traditional lab tests. This resonates with patients and healthcare providers alike, driving POCT adoption. Technological advancements play a crucial role, with microfluidics, lateral flow assays, and biosensors paving the way for more accurate, user-friendly, and rapid POCT devices. This empowers early diagnosis and swift treatment initiation, potentially improving patient outcomes. However, challenges remain. Lack of comprehensive reimbursement
Point-of-Care Testing for Erythrodermic Psoriasis Market Drivers:
Increased Demand for Rapid Diagnosis is driving market growth.
Erythrodermic Psoriasis, unlike its milder counterparts, isn't just scaly skin; it's a potentially life-threatening condition demanding immediate action. Imagine the agony of waiting days for traditional lab tests while your body burns and flares. This agonizing delay can worsen the condition and jeopardize health. Here's where Point-of-Care Testing (POCT) steps in as a game-changer. Instead of days, POCT delivers precise results in minutes, right at the doctor's office. This lightning-fast diagnosis paves the way for immediate treatment, potentially halting the disease's progression and significantly improving outcomes. Think of it as the difference between battling a fire with buckets of water versus a high-powered hose – faster, more effective, and potentially life-saving. This rapid response isn't just about convenience; it translates to better health. Early intervention can minimize the risk of complications like infections and heart problems, leading to improved long-term health and well-being.
Growing Awareness and Prevalence is fueling demand for PCOT.
The spotlight on Psoriasis is shining brighter, leading to a double-edged sword for Erythrodermic Psoriasis. Increased awareness means earlier diagnoses of milder Psoriasis, but it also unveils more hidden cases of the severe Erythrodermic form. This potential rise in identifications, coupled with the already growing prevalence of Psoriasis itself, paints a picture of heightened demand for efficient diagnostic tools. Traditional lab tests, with their agonizing wait times, simply won't cut it. Enter POCT, the knight in shining armor promising results within minutes, right at the doctor's office. This swift diagnosis acts as a springboard for immediate treatment, potentially preventing the condition from spiraling out of control. Think of it as nipping the bud before it blooms into a full-blown crisis. POCT doesn't just offer convenience; it offers a crucial window of opportunity, potentially safeguarding the health and well-being of individuals facing this potentially life-threatening condition.
Development of more accurate, reliable, and user-friendly POCT devices specifically for Erythrodermic Psoriasis is increasing.
The fight against Erythrodermic Psoriasis is getting a tech boost! New-age POCT devices are being specifically designed for this severe condition, packing accuracy, reliability, and user-friendliness into a punch. Imagine skipping lengthy lab waits and getting results within minutes, right at your doctor's office. These advancements promise faster diagnoses, leading to swifter treatment and potentially better patient outcomes. Think of it as a revolution in diagnosis, empowering doctors to act quickly and effectively against this serious condition. The future of Erythrodermic Psoriasis diagnosis is looking brighter, thanks to these innovative POCT devices.
Point-of-Care Testing for Erythrodermic Psoriasis Market challenges and restraints
Currently, there are few POCT devices specifically designed for Erythrodermic Psoriasis diagnosis. This limited availability restricts patient access and slows market growth
The scarcity of POCT devices specifically designed for Erythrodermic Psoriasis creates a double whammy - restricted patient access and sluggish market growth. Patients endure agonizing delays, potentially worsening their condition, while the market struggles to meet the rising demand for swift and accurate diagnosis. It's a race against time, and the lack of POCT options puts everyone at a disadvantage. Thankfully, the tide is turning with ongoing research and development. Hopefully, wider availability of POCT devices will soon bridge this gap, offering timely diagnosis and hope for better patient outcomes.
POCT faces competition from traditional lab testing, which is well-established and has lower upfront costs
Point-of-care testing (POCT) for Erythrodermic Psoriasis faces a formidable foe: traditional lab testing. Deeply entrenched with established infrastructure and lower upfront costs, lab testing presents a significant hurdle for POCT adoption. The convenience and speed of POCT are undeniable, but convincing healthcare providers and patients to switch requires overcoming cost concerns. Reimbursement challenges further complicate matters, as POCT might face stricter scrutiny compared to well-established lab processes. While POCT manufacturers strive to reduce costs and secure favorable reimbursement policies, the battle for market share remains fierce. Ultimately, the victor will be determined by balancing affordability with the unique value proposition of POCT – faster diagnosis, improved patient experience, and potentially better outcomes. Only time will tell if POCT can overcome these challenges and carve its niche in the Erythrodermic Psoriasis testing landscape.
Lack of standardized POCT protocols and test procedures can create inconsistencies and confusion, hindering wider adoption in clinical settings
The promise of POCT for Erythrodermic Psoriasis is dimmed by the lack of a unified rulebook. Inconsistent testing procedures and protocols create a confusing mess for healthcare providers, hindering wider adoption. Imagine doctors struggling to interpret results due to variations in POCT devices and procedures. This inconsistency breeds uncertainty and makes it difficult to trust the accuracy of diagnoses, creating a major roadblock for POCT integration into clinical settings. Standardization is urgently needed to bridge this gap, ensuring reliable and comparable results across different POCT devices and facilities. Only then can POCT truly live up to its potential and revolutionize the diagnosis of this serious condition.
Market Opportunities
Erythrodermic psoriasis presents a compelling market opportunity for POCT solutions. The rising prevalence of psoriasis and limitations of traditional testing methods fuel demand for faster, more convenient diagnostics. POCT shines here, offering rapid results, improved patient experience, and potential cost savings. Advancements in microfluidics, biosensors, and lateral flow assays pave the way for accurate, user-friendly devices, enabling early diagnosis and swift treatment. However, challenges like limited reimbursement and regulatory hurdles require solutions. The key lies in developing cost-effective devices, navigating regulations efficiently, and training healthcare personnel. By addressing these, POCT players can unlock significant opportunities in this growing market, ultimately improving patient outcomes and healthcare delivery.
POINT OF CARE TESTING FOR ERYTHRODERMIC PSORIASIS MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
12.2% |
|
Segments Covered |
By Test Type, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Becton Dickinson (BD), Quidel Corporation |
Point of Care Testing for Erythrodermic Psoriasis Market Segmentation - By Test Type
-
Diagnostic tests
-
Monitoring tests
-
Differential diagnosis tests
Currently, diagnostic tests hold the lion's share of the POCT market for Erythrodermic Psoriasis. The urgency of a definitive diagnosis in this severe condition makes tests that quickly confirm or rule out Erythrodermic Psoriasis highly sought-after by both patients and healthcare professionals. While monitoring and differential diagnosis tests are crucial for ongoing management and accurate treatment, their demand lags behind the immediate need for a clear initial diagnosis. This dynamic is likely to shift as POCT technology advances and the market matures, making monitoring and differential diagnosis tests more accessible and user-friendly, potentially increasing their future prevalence.
Point of Care Testing for Erythrodermic Psoriasis Market Segmentation - Regional Analysis
-
North America
-
Asia-Pacific
-
Europe
-
South America
-
Middle East and Africa
North America and Europe are the dominant nations in this market, driven by high healthcare spending, established infrastructure, and a strong research community. While Asia-Pacific is the fastest-growing region. Asia has significant potential due to its vast population and rising healthcare investments. Africa, Latin America, and the Middle East are the slowest-growing regions.
COVID-19 Impact Analysis on the Point-of-Care Testing for Erythrodermic Psoriasis Market
The COVID-19 pandemic's impact on the erythrodermic psoriasis market is a mixed bag. While disrupted healthcare access, stress, and treatment disruptions posed challenges, the pandemic also brought opportunities. Increased awareness, telehealth adoption, a focus on home-based care, and potential research breakthroughs fueled by increased funding could drive long-term market growth. Navigating this complex landscape requires considering regional variations, new COVID-19 variants, and vaccine rollout's impact on healthcare access. Overall, the erythrodermic psoriasis market faces both hurdles and promising possibilities in the post-pandemic era
Latest trends/Developments
The POCT landscape for Erythrodermic Psoriasis is buzzing with promising developments, despite facing challenges. Recognizing the urgent need for faster diagnosis, researchers are actively developing new POCT devices specifically designed for this severe condition. These devices focus on accuracy, reliability, and user-friendliness, aiming to provide quick results directly in doctor's offices. Miniaturization and automation technologies are making these devices more portable and easier to operate, even in resource-constrained settings. Additionally, efforts are underway to standardize testing procedures and protocols, ensuring consistent and reliable results across different devices. While limited availability, reimbursement concerns, and competition from traditional lab testing remain hurdles, ongoing research, collaborations, and regulatory support are paving the way for wider adoption of POCT. This could revolutionize the diagnosis of Erythrodermic Psoriasis, enabling swifter interventions and potentially improving patient outcomes.
Key Players:
-
Abbott Laboratories
-
Roche Diagnostics
-
Siemens Healthineers
-
Becton Dickinson (BD)
-
Quidel Corporation
Chapter 1. POINT OF CARE TESTING FOR ERYTHRODERMIC PSORIASIS MARKET – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. POINT OF CARE TESTING FOR ERYTHRODERMIC PSORIASIS MARKET – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. POINT OF CARE TESTING FOR ERYTHRODERMIC PSORIASIS MARKET – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. POINT OF CARE TESTING FOR ERYTHRODERMIC PSORIASIS MARKET Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. POINT OF CARE TESTING FOR ERYTHRODERMIC PSORIASIS MARKET – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. POINT OF CARE TESTING FOR ERYTHRODERMIC PSORIASIS MARKET – By Test Type
6.1 Introduction/Key Findings
6.2 Diagnostic tests
6.3 Monitoring tests
6.4 Differential diagnosis tests
6.5 Y-O-Y Growth trend Analysis By Test Type
6.6 Absolute $ Opportunity Analysis By Test Type, 2024-2030
Chapter 7. POINT OF CARE TESTING FOR ERYTHRODERMIC PSORIASIS MARKET , By Geography – Market Size, Forecast, Trends & Insights
7.1 North America
7.1.1 By Country
7.1.1.1 U.S.A.
7.1.1.2 Canada
7.1.1.3 Mexico
7.1.2 By Test Type
7.1.3 Countries & Segments - Market Attractiveness Analysis
7.2 Europe
7.2.1 By Country
7.2.1.1 U.K
7.2.1.2 Germany
7.2.1.3 France
7.2.1.4 Italy
7.2.1.5 Spain
7.2.1.6 Rest of Europe
7.2.2 By Test Type
7.2.3 Countries & Segments - Market Attractiveness Analysis
7.3 Asia Pacific
7.3.1 By Country
7.3.1.1 China
7.3.1.2 Japan
7.3.1.3 South Korea
7.3.1.4 India
7.3.1.5 Australia & New Zealand
7.3.1.6 Rest of Asia-Pacific
7.3.2 By Test Type
7.3.3 Countries & Segments - Market Attractiveness Analysis
7.4 South America
7.4.1 By Country
7.4.1.1 Brazil
7.4.1.2 Argentina
7.4.1.3 Colombia
7.4.1.4 Chile
7.4.1.5 Rest of South America
7.4.2 By Test Type
7.4.3 Countries & Segments - Market Attractiveness Analysis
7.5 Middle East & Africa
7.5.1 By Country
7.5.1.1 United Arab Emirates (UAE)
7.5.1.2 Saudi Arabia
7.5.1.3 Qatar
7.5.1.4 Israel
7.5.1.5 South Africa
7.5.1.6 Nigeria
7.5.1.7 Kenya
7.5.1.8 Egypt
7.5.1.9 Rest of MEA
7.5.2 By Test Type
7.5.3 Countries & Segments - Market Attractiveness Analysis
Chapter 8. POINT OF CARE TESTING FOR ERYTHRODERMIC PSORIASIS MARKET – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
8.1 Abbott Laboratories
8.2 Roche Diagnostics
8.3 Siemens Healthineers
8.4 Becton Dickinson (BD)
8.5 Quidel Corporation
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The global point-of-care testing (POCT) market size was estimated at USD 37.03 billion in the year 2023 and is expected to reach around USD 82.8 billion by 2030 with an increasing CAGR of 12.2% from 2023 to 2030 due to increasing demands from the market due to increasing disorders among the population
Increased Demand for Rapid Diagnosis, Growing Awareness and Prevalence, and Development of more accurate, reliable, and user-friendly POCT devices specifically for Erythrodermic Psoriasis are increasing these are the reasons which are driving the market.
Based on test type it is divided into three segments – Diagnostic tests, Monitoring tests, Differential diagnosis tests
North America is the most dominant region for the Erythrodermic Psoriasis Market.
Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Becton Dickinson (BD), Quidel Corporation.