Chapter 1. PERIPHERAL VASCULAR ACCESS ARKET – Scope & Methodology
1.1. Market Segmentation
1.2. Assumptions
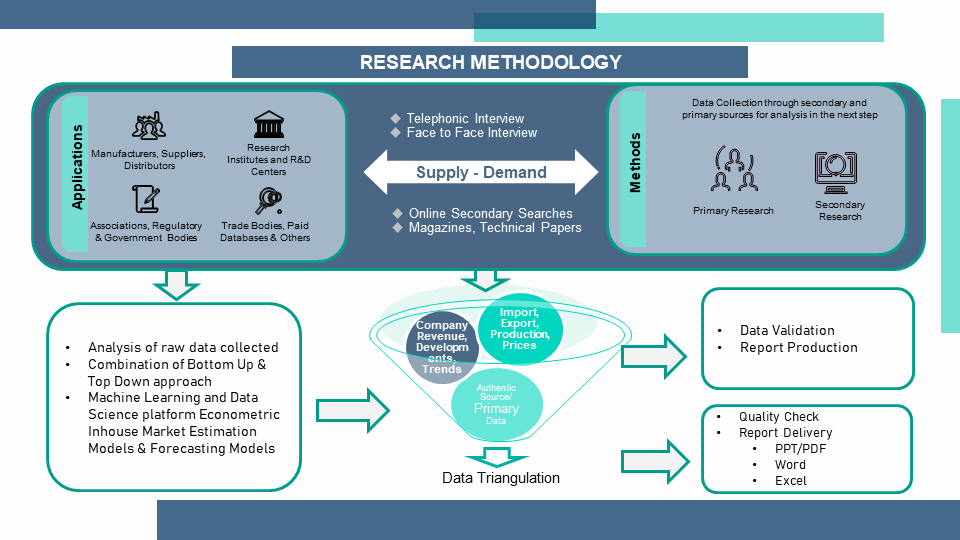
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2. PERIPHERAL VASCULAR ACCESS MARKET – Executive Summary
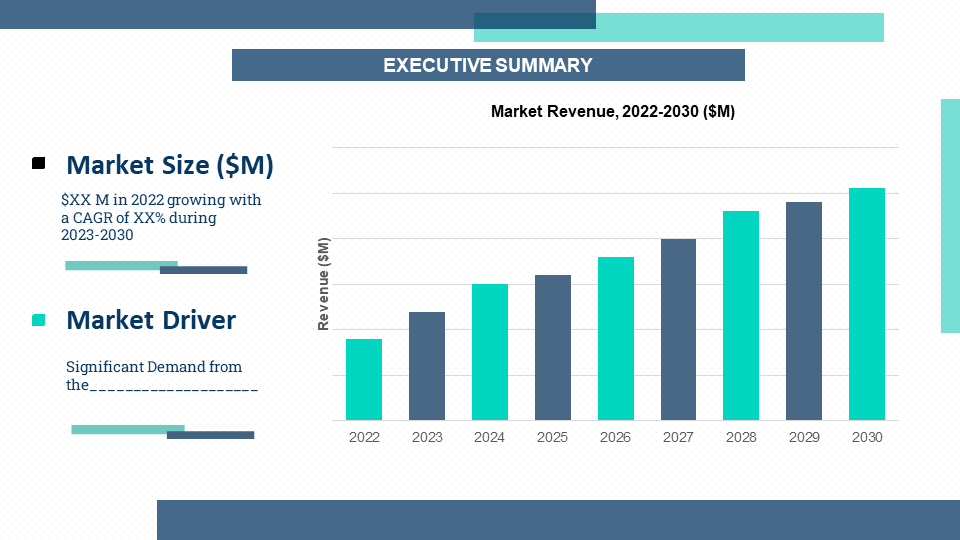
2.1. Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-19 Impact Analysis
2.3.1. Impact during 2023 – 2030
2.3.2. Impact on Supply – Demand
Chapter 3. PERIPHERAL VASCULAR ACCESS MARKET – Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4. PERIPHERAL VASCULAR ACCESS MARKET - Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatory Scenario - By Region
4.3 Customer Analysis
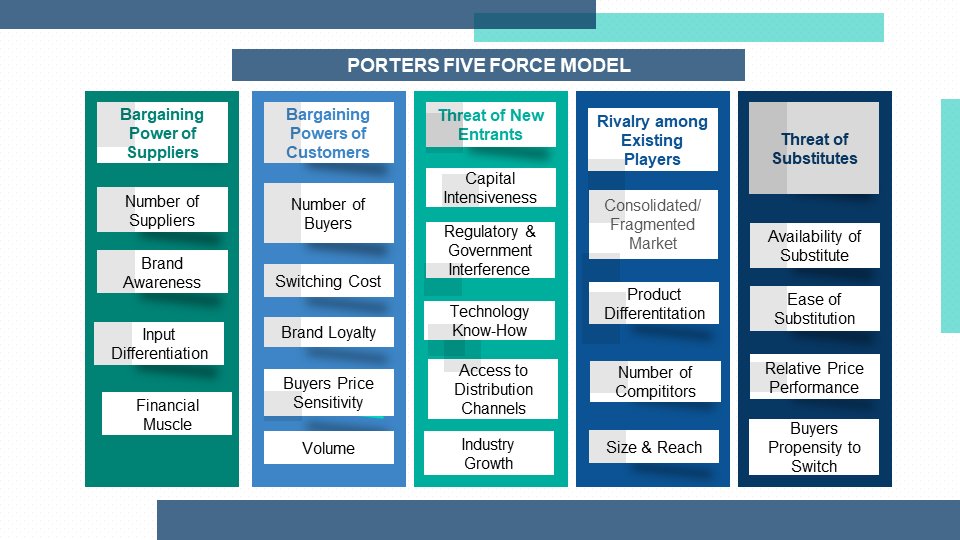
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. PERIPHERAL VASCULAR ACCESS MARKET - Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
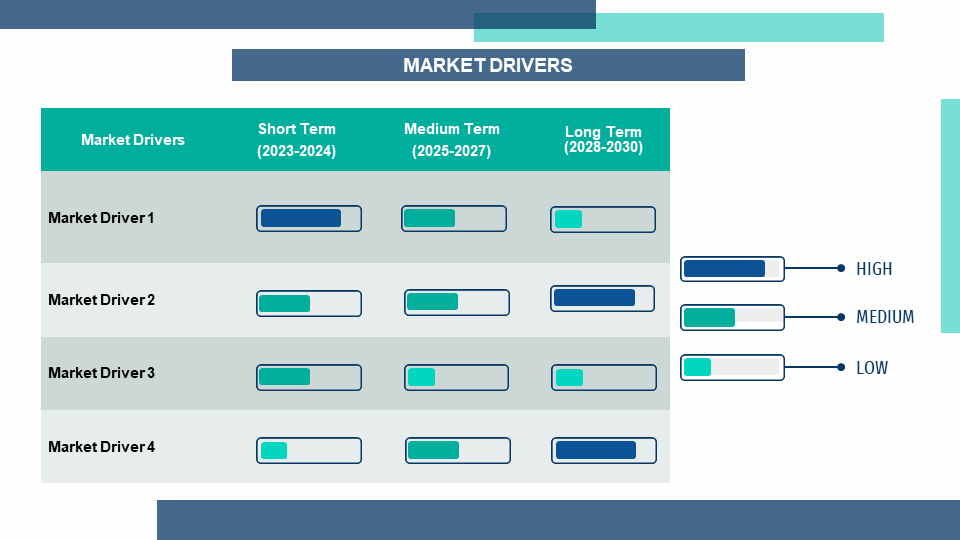
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6. PERIPHERAL VASCULAR ACCESS MARKET –By Type
6.1. Peripheral Stents
6.1.1. Iliac Artery Stents
6.1.2. Femoral Artery Stents
6.1.3. Carotid Artery Stents
6.1.4. Renal Artery Stents
6.1.5. Other Peripheral Stents
6.2. PTA Balloons
6.3. Catheters
6.3.1. Angiography Catheters
6.3.2. Guiding Catheters
6.3.3. IVUS/OCT Catheters
6.4. Endovascular Aneurysm Repair Stent Grafts
6.4.1. Thoracic Endovascular Aneurysm Stent Grafts
6.4.2. Abdominal Endovascular Aneurysm Stent Grafts
6.5. Plaque Modification Devices
6.5.1. Atherectomy Devices
6.5.2. Thrombectomy Devices
6.6. Peripheral Accessories
6.6.1. Guidewires
6.6.1.1. Workhorse Guidewires
6.6.1.2. Specialty Guidewires
6.6.1.3. Extra Support Guidewires
6.6.1.4. Frontline Finesse Guidewires
6.6.2. Peripheral Vascular Closure Devices
6.6.3. Balloon Inflation Devices
6.6.4. Introducer Sheaths
6.7. Inferior Vena Cava Filters
6.7.1. Permanent Filters
6.7.2. Retrievable Filters
6.8. Hemodynamic Flow Alteration Devices
6.8.1. Chronic Total Occlusion Devices
6.8.2. Embolic Protection Devices
Chapter 7. PERIPHERAL VASCULAR ACCESS MARKET – By Region
7.1. North America
7.2. Europe
7.3.The Asia Pacific
7.4.Latin America
7.5. Middle-East and Africa
Chapter 8. PERIPHERAL VASCULAR ACCESS MARKET– Company Profiles – (Overview, Product Portfolio, Financials, Developments)
8.1. Company 1
8.2. Company 2
8.3. Company 3
8.4. Company 4
8.5. Company 5
8.6. Company 6
8.7. Company 7
8.8. Company 8
8.9. Company 9
8.10. Company 10
Download Sample
Choose License Type
2500
4250
5250
6900



