Global Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market Size (2024-2030)
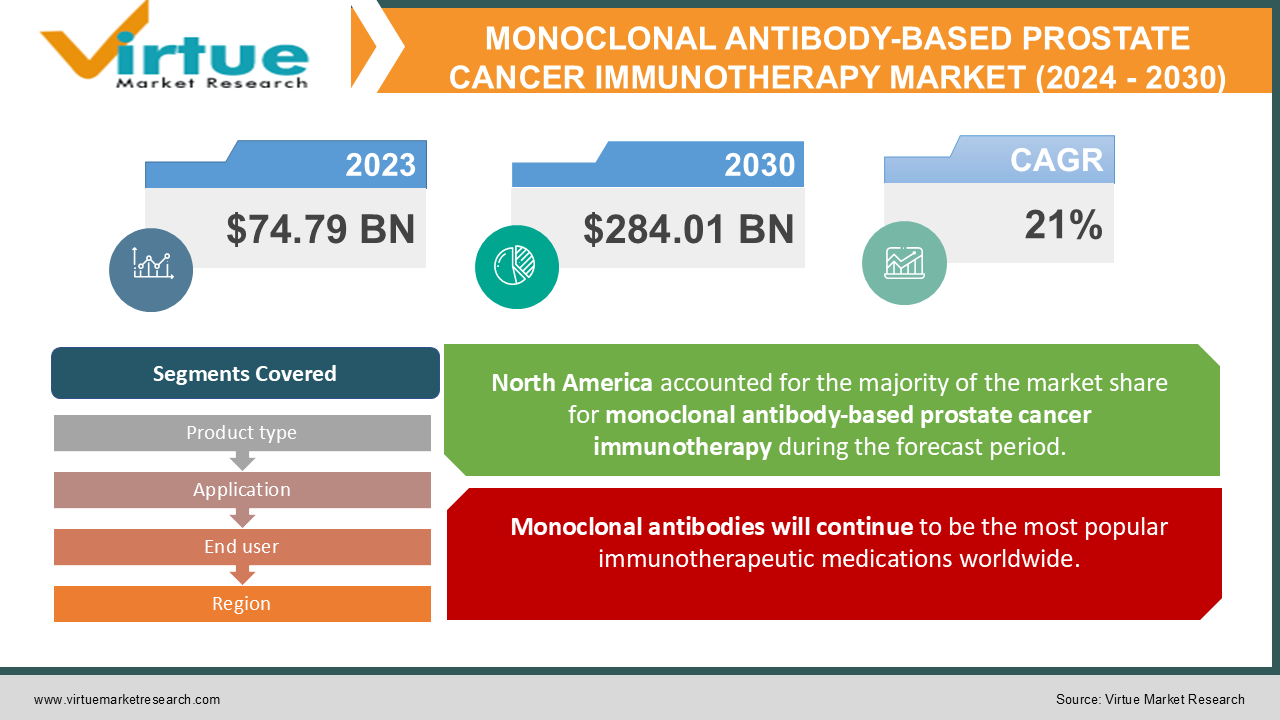
The Global Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market was estimated to be worth USD 74.79 Billion in 2023 and is projected to reach a value of USD 284.01 Billion by 2030, growing at a CAGR of 21% during the forecast period 2024-2030.
Monoclonal antibodies, a type of synthetic protein, are used in immunotherapy, a treatment for prostate cancer. These antibodies can bind to particular locations on or near prostate cancer cells, which can then be used to deliver a toxic substance, stop the cancer cells from growing, or start an immune reaction against them. Targeting different molecules implicated in prostate cancer, monoclonal antibodies can be used either in isolation or in conjunction with other medical treatments such as radiation, chemotherapy, or additional immunotherapies. Treatment with monoclonal antibodies has the benefit of being able to stimulate the immune system, precisely target the target, and stay in the bloodstream for a longer period. Monoclonal antibody therapies have the potential to dramatically improve the quality of life for a large number of people worldwide, both now and in the future.
Key Market Insights:
Monoclonal antibody-based cancer immunotherapy is expected to grow at a rate of 9% per year, making it a popular option for treatment. These treatments have the exceptional capacity to boost the immune system's ability to combat cancer. Monoclonal antibodies function by directing the immune system to attack cancer cells, rupturing the membranes of cancerous cells, delaying the growth of cancerous cells, preventing the development of blood vessels that sustain tumors, enabling chemotherapy, and creating links between immune cells and cancer cells. All things considered, these therapies have a number of advantages in the fight against cancer.
Global Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market Drivers:
Monoclonal antibodies will continue to be the most popular immunotherapeutic medications worldwide.
Numerous treatment options, including immune checkpoint inhibitors, cancer vaccines, PD-1/PD-L1, CTLA-4, immune system modulators, and monoclonal antibodies, are available in the global market for cancer immunotherapy. Monoclonal antibodies accounted for a sizeable portion of the market in 2021 and were widely used. Monoclonal antibodies are the most commonly prescribed option among the various immunotherapy options. Blincyto (blinatumomab), a bispecific antibody created by Amgen to treat B-cell lymphoblastic leukemia, is one prominent example. Because they work better and are less expensive than other treatments, monoclonal antibodies are now the treatment of choice. New options for treating cancer have been found as a result of ongoing research and development in this field. These options include conjugated, bispecific, and naked antigen-binding monoclonal antibodies.
Prostate cancer, one of the most common malignancies in men worldwide, is becoming more common.
One kind of cancer that can arise in the prostate gland, which is a component of the male reproductive system, is prostate cancer. Urinary problems, erectile dysfunction, blood in the urine or semen, and bone pain are possible symptoms. Prostate cancer risk factors include age, ethnicity, family history, genetics, lifestyle, and exposure to the environment. Males over 50 are more likely to get it, and certain genetic factors or a family history of prostate cancer may raise the risk. Prostate cancer is becoming more common, so safe and effective treatments are required to increase patient survival and quality of life. One method that targets specific substances expressed by prostate cancer cells and either inhibits, poisons, or aids the immune system in attacking the cancer cells is called monoclonal antibody-based therapy.
Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market Challenges and Restraints:
Patients may have adverse reactions, sometimes referred to as infusion reactions, following monoclonal antibody-based therapy. Allergies, rash, fatigue, fever, nausea, diarrhea, and infections are some examples of these. Depending on the type and dosage of therapy, the patient's health, the treatment schedule, and other variables, the severity of these reactions can range from mild to severe or even life-threatening. Anaphylaxis, serum sickness, tumor lysis syndrome, and cytokine release syndrome are a few of the potential causes of these negative effects. Immune-related toxicities can also impact various organs and systems, including the nervous system, endocrine glands, skin, gastrointestinal tract, liver, lungs, and blood cells. The manifestation of these toxicities during or after therapy may vary, necessitating further medical intervention.
Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market Opportunities:
The World Health Organization (WHO) estimates that about one-third of cancer-related deaths are caused by variables such as high body mass index, low fruit and vegetable diet, alcohol consumption, and lack of physical activity. Governments around the world are investing heavily in laboratories to produce efficient monoclonal antibodies for cancer treatment due to the rising incidence of cancer. Additionally, more monoclonal antibodies are being approved by governments, which presents the market with a wealth of opportunities. According to a study, the U.S. FDA has approved more than 100 monoclonal antibodies to treat different types of cancer, suggesting that the market is trending positively.
MONOCLONAL ANTIBODY-BASED PROSTATE CANCER IMMUNOTHERAPY MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
21% |
|
Segments Covered |
By Product type, Application, End user, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Amgen, Inc., NOVARTIS AG, GlaxoSmithKline plc, ELI LILY AND COMPANY, Pfizer, Inc., BAYER AG, Merck & Co, Janssen Biotech, Bristol-Myers Squibb Company, Spectrum Pharmaceuticals |
Global Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market Segmentation: By Product Type
-
Anti- Prostate Specific Membrane Antigen (PSMA) Monoclonal Antibody-Based
-
Anti- Cytotoxic T-Lymphocyte Associated Antigen 4 (CTLA-4) Monoclonal Antibody-Based
-
Anti-Programmed Cell Death Protein 1 PD-1/ligand PD-L1(PD-1/PD-L1) Monoclonal Antibody-Based
-
Others
In monoclonal antibody-based therapy, Anti-Programmed Cell Death Protein 1 PD-1/ligand PD-L1 (PD-1/PD-L1) is the category growing at the fastest rate. The reason for this growth is that PD-1/PD-L1 inhibitors have proven effective in treating a variety of cancers, including prostate cancer. By preventing the interaction between PD-1 in immune cells and PD-L1 in cancer cells, these inhibitors improve the immune system's ability to recognize and combat cancer cells. The market leader is the Anti-Prostate Specific Membrane Antigen (PSMA) Monoclonal Antibody-Based segment. Prostate cancer cells have high levels of the protein PSMA on their surface, which makes it a desirable target for immunotherapy. Targeting PSMA, monoclonal antibodies can specifically target prostate cancer cells, stimulating the immune system to fight cancer cells or delivering therapeutic drugs. The prevalence of PSMA use in prostate cancer immunotherapy research and the rise in clinical trials investigating PSMA-targeted monoclonal antibodies are the main factors driving this segment's dominance.
Global Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market Segmentation: By Application
-
Early-Stage prostate cancer
-
Metastatic prostate cancer
-
Castration-resistant prostate cancer
Metastatic prostate cancer, or prostate cancer that has spread to other parts of the body such as the liver, lungs, lymph nodes, or bones, is predicted to grow at the fastest rate in the market. The rising prevalence and death rate from metastatic prostate cancer, the inadequacy of conventional therapies, and the development of novel monoclonal antibodies (mAbs) that target metastases and enhance patient outcomes are all blamed for this expansion. Lutitudinium-177-PSMA-617, pembrolizumab, nivolumab, atezolizumab, and enoblituzumab are a few mAbs for metastatic prostate cancer. Castration-resistant prostate cancer (CRPC), which advances despite hormonal treatment, is anticipated to lead the market in terms of application. This is because CRPC is highly prevalent and has a poor prognosis. It is also because there are approved mAbs for CRPC and there is a high demand for these treatments. A few mAbs for CRPC are enoblituzumab, atezolizumab, nivolumab, pembrolizumab, ipilimumab, and Sipuleucel-T.
Global Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market Segmentation: By End user
-
Hospitals and clinics
-
Cancer research centers
-
Academic and research institutes
It is anticipated that academic and research institutes—that is, entities engaged in the investigation and instruction of monoclonal antibodies (mAbs) for the immunotherapy of prostate cancer—will expand quickly. This is because there is now more money and support available for mAb research from a variety of sources, such as philanthropic donors, NGOs, government agencies, and business partners. The Parker Institute for Cancer Immunotherapy, Stanford University, University of California San Francisco, Johns Hopkins University, National Cancer Institute, and Cancer Research UK are a few examples. It is anticipated that the most common end-user category will be hospitals and clinics, which are healthcare facilities that use mAbs for prostate cancer diagnosis and treatment. This is a result of the widespread prescription and application of mAbs in these contexts, the availability of qualified healthcare professionals, the presence of trained healthcare professionals, the observance of quality and safety standards, reimbursement policies, and government support.
Global Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market Segmentation: By Region
-
North America
-
Europe
-
Asia-Pacific
-
Middle East and Africa
-
South America
Because of its developed healthcare system, more government programs, and an increasing number of cancer patients, North America is anticipated to be a profitable market for cancer immunotherapy. Thanks to the advancement of bioinformatics tools for drug development, the region is at the forefront of immunotherapy use. Over the projection period, Asia Pacific is expected to grow at the fastest rate. China and Japan are running a number of clinical trials and have introduced novel immunotherapy drugs that have been approved by the FDA. The region's increasing use of immunotherapy to treat tumors is being aided by the approval of these novel drugs in China and Japan.
COVID-19 Impact on the Global Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market:
Both beneficial and negative effects of the COVID-19 pandemic have been felt in the market for prostate cancer treatments based on monoclonal antibodies. Positively, the pandemic has spurred interest in the study and creation of monoclonal antibodies to treat a range of illnesses, including COVID-19. Certain COVID-19 antibodies, such as sotrovimab, imdevimab, and basiliximab, may also be useful in treating prostate cancer, which would increase demand for and awareness of these therapies. Nevertheless, difficulties have emerged, such as delays and shortages caused by disruptions in the monoclonal antibody supply chain and manufacturing. Prostate cancer clinical trial progress has also been hindered, and data quality has been compromised. Furthermore, the pandemic has made prostate cancer patients' expenses and stress levels higher, which may restrict their ability to obtain and adhere to monoclonal antibody-based therapy.
Latest Trend/Development:
The FDA in the United States approved Lutathera as a treatment for advanced prostate cancer that has not responded to prior treatments. A radiolabeled anti-PSMA monoclonal antibody is called Lutathera. The anti-PD-1 monoclonal antibody Jemperli received accelerated approval from the U.S. FDA for the treatment of advanced or recurrent endometrial cancer that has not responded to prior platinum-containing chemotherapy. This approval is limited to tumors that exhibit specific genetic characteristics, such as high microsatellite instability or a deficiency in mismatch repair.
Key Players:
-
Amgen, Inc.
-
NOVARTIS AG
-
GlaxoSmithKline plc
-
ELI LILY AND COMPANY
-
Pfizer, Inc.
-
BAYER AG
-
Merck & Co
-
Janssen Biotech
-
Bristol-Myers Squibb Company
-
Spectrum Pharmaceuticals
Chapter 1. Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market – By End-User
6.1 Introduction/Key Findings
6.2 Hospitals and clinics
6.3 Cancer research centers
6.4 Academic and research institutes
6.5 Y-O-Y Growth trend Analysis By End-User
6.6 Absolute $ Opportunity Analysis By End-User, 2024-2030
Chapter 7. Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market – By Product Type
7.1 Introduction/Key Findings
7.2 Anti- Prostate Specific Membrane Antigen (PSMA) Monoclonal Antibody-Based
7.3 Anti- Cytotoxic T-Lymphocyte Associated Antigen 4 (CTLA-4) Monoclonal Antibody-Based
7.4 Anti-Programmed Cell Death Protein 1 PD-1/ligand PD-L1(PD-1/PD-L1) Monoclonal Antibody-Based
7.5 Others
7.6 Y-O-Y Growth trend Analysis By Product Type
7.7 Absolute $ Opportunity Analysis By Product Type, 2024-2030
Chapter 8. Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market – By Application
8.1 Introduction/Key Findings
8.2 Early-Stage prostate cancer
8.3 Metastatic prostate cancer
8.4 Castration-resistant prostate cancer
8.5 Y-O-Y Growth trend Analysis By Application
8.6 Absolute $ Opportunity Analysis By Application, 2024-2030
Chapter 9. Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market , By Geography – Market Size, Forecast, Trends & Insights
9.1 North America
9.1.1 By Country
9.1.1.1 U.S.A.
9.1.1.2 Canada
9.1.1.3 Mexico
9.1.2 By End-User
9.1.3 By Product Type
9.1.4 By By Application
9.1.5 Countries & Segments - Market Attractiveness Analysis
9.2 Europe
9.2.1 By Country
9.2.1.1 U.K
9.2.1.2 Germany
9.2.1.3 France
9.2.1.4 Italy
9.2.1.5 Spain
9.2.1.6 Rest of Europe
9.2.2 By End-User
9.2.3 By Product Type
9.2.4 By Application
9.2.5 Countries & Segments - Market Attractiveness Analysis
9.3 Asia Pacific
9.3.1 By Country
9.3.1.1 China
9.3.1.2 Japan
9.3.1.3 South Korea
9.3.1.4 India
9.3.1.5 Australia & New Zealand
9.3.1.6 Rest of Asia-Pacific
9.3.2 By End-User
9.3.3 By Product Type
9.3.4 By Application
9.3.5 Countries & Segments - Market Attractiveness Analysis
9.4 South America
9.4.1 By Country
9.4.1.1 Brazil
9.4.1.2 Argentina
9.4.1.3 Colombia
9.4.1.4 Chile
9.4.1.5 Rest of South America
9.4.2 By End-User
9.4.3 By Product Type
9.4.4 By Application
9.4.5 Countries & Segments - Market Attractiveness Analysis
9.5 Middle East & Africa
9.5.1 By Country
9.5.1.1 United Arab Emirates (UAE)
9.5.1.2 Saudi Arabia
9.5.1.3 Qatar
9.5.1.4 Israel
9.5.1.5 South Africa
9.5.1.6 Nigeria
9.5.1.7 Kenya
9.5.1.8 Egypt
9.5.1.9 Rest of MEA
9.5.2 By End-User
9.5.3 By Product Type
9.5.4 By Application
9.5.5 Countries & Segments - Market Attractiveness Analysis
Chapter 10. Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
10.1 AB Sciex
10.2 Advion Inc.
10.3 Agilent Technologies
10.4 Bruker Corporation
10.5 Extrel CMS LLC
10.6 Hitachi High-Tech Corporation
10.7 JEOL Ltd.
10.8 Kore Technology Ltd.
10.9 LECO Corporation
10.10 Markes International Ltd.
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
Prostate cancer treated with monoclonal antibodies, a form of treatment known as immunotherapy employs synthetic proteins that can attach to particular sites on or around prostate cancer cells to either impede their activity, deliver a harmful chemical, or elicit an immune response.
The Global Monoclonal Antibody-Based Prostate Cancer Immunotherapy Market was estimated to be worth USD 74.79 Billion in 2023 and is projected to reach a value of USD 284.01 Billion by 2030, growing at a CAGR of 21% during the forecast period 2024-2030.
The rising incidence of prostate cancer one of the most frequent cancers in males in the world and Monoclonal Antibodies Remain the Dominant Immunotherapeutic Drugs in the Global Market
Amgen, Inc., NOVARTIS AG, GlaxoSmithKline plc, ELI LILY AND COMPANY, Pfizer, Inc., BAYER AG, Merck & Co, Janssen Biotech, Bristol-Myers Squibb Company, Spectrum Pharmaceuticals
The possible side effects and immune-related toxicities of prostate cancer monoclonal antibody-based therapy