Chapter 1. MEDICAL DEVICE OUTSOURCING MARKET – Scope & Methodology
1.1. Market Segmentation
1.2. Assumptions
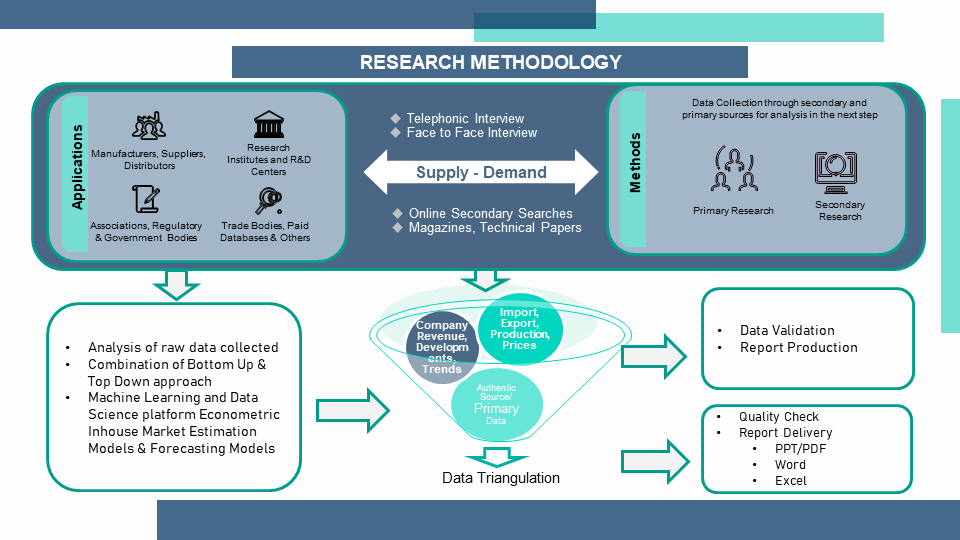
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2. MEDICAL DEVICE OUTSOURCING MARKET – Executive Summary
2.1. Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-19 Impact Analysis
2.3.1. Impact during 2023 – 2030
2.3.2. Impact on Supply – Demand
Chapter 3. MEDICAL DEVICE OUTSOURCING MARKET – Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4. MEDICAL DEVICE OUTSOURCING MARKET - Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatory Scenario - By Region
4.3 Customer Analysis
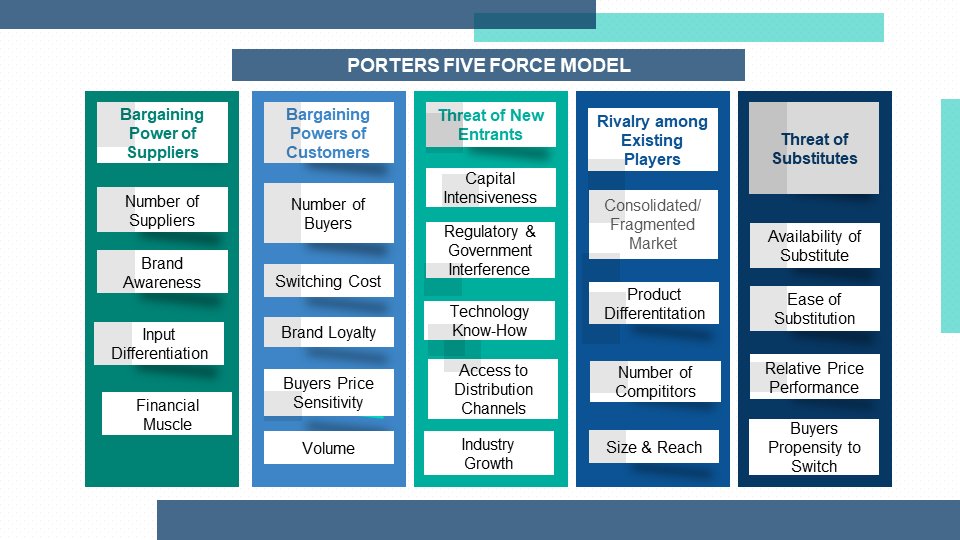
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. MEDICAL DEVICE OUTSOURCING MARKET - Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6. MEDICAL DEVICE OUTSOURCING MARKET – By Service
6.1. Quality Assurance
6.2. Regulatory Affairs Services
6.2.1. Clinical trials applications and product registrations
6.2.2. Regulatory writing and publishing
6.2.3. Legal representation
6.2.4. Other
6.3. Product Design and Development Services
6.3.1. Designing & Engineering
6.3.2. Machining
6.3.3. Molding
6.3.4. Packaging
6.4. Product Testing & Sterilization Services
6.5. Product Implementation Services
6.6. Product Upgrade Services
6.7. Product Maintenance Services
6.8. Contract Manufacturing
6.8.1. Accessories Manufacturing
6.8.2. Assembly Manufacturing
6.8.3. Component Manufacturing
6.8.4. Device Manufacturing
Chapter 7. MEDICAL DEVICE OUTSOURCING MARKET – By Application
7.1 Cardiology
7.1.1. Class I
7.1.2. Class II
7.1.3. Class III
7.2. Diagnostic imaging
7.2.1. Class I
7.2.2. Class II
7.2.3. Class III
7.3. Orthopedic
7.3.1. Class I
7.3.2. Class II
7.3.3. Class III
7.4. IVD
7.4.1. Class I
7.4.2. Class II
7.4.3. Class III
7.5. Ophthalmic
7.5.1. Class I
7.5.2. Class II
7.5.3. Class III
7.6. General and plastic surgery
7.6.1. Class I
7.6.2. Class II
7.6.3. Class III
7.7. Drug delivery
7.7.1. Class I
7.7.2. Class II
7.7.3. Class III
7.8.Dental
7.8.1. Class I
7.8.2. Class II
7.8.3. Class III
7.9. Endoscopy
7.9.1. Class I
7.9.2. Class II
7.9.3. Class III
7.10 Diabetes care
7.10.1. Class I
7.10.2. Class II
7.10.3. Class III
7.11. Others
7.11.1. Class I
7.11.2. Class II
7.11.3. Class III
Chapter 8. MEDICAL DEVICE OUTSOURCING MARKET – By Class
8.1 Class I
8.2. Class II
8.3. Class III
Chapter 9. MEDICAL DEVICE OUTSOURCING MARKET – By Region
9.1. North America
9.2. Europe
9.3.The Asia Pacific
9.4.Latin America
9.5. Middle-East and Africa
Chapter 10. MEDICAL DEVICE OUTSOURCING MARKET– Company Profiles – (Overview, Product Portfolio, Financials, Developments)
10.1. Company 1
10.2. Company 2
10.3. Company 3
10.4. Company 4
10.5. Company 5
10.6. Company 6
10.7. Company 7
10.8. Company 8
10.9. Company 9
10.10. Company 10
Download Sample
Choose License Type
2500
4250
5250
6900



