GLOBAL ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY (INAAT) MARKET SIZE (2024 - 2030)
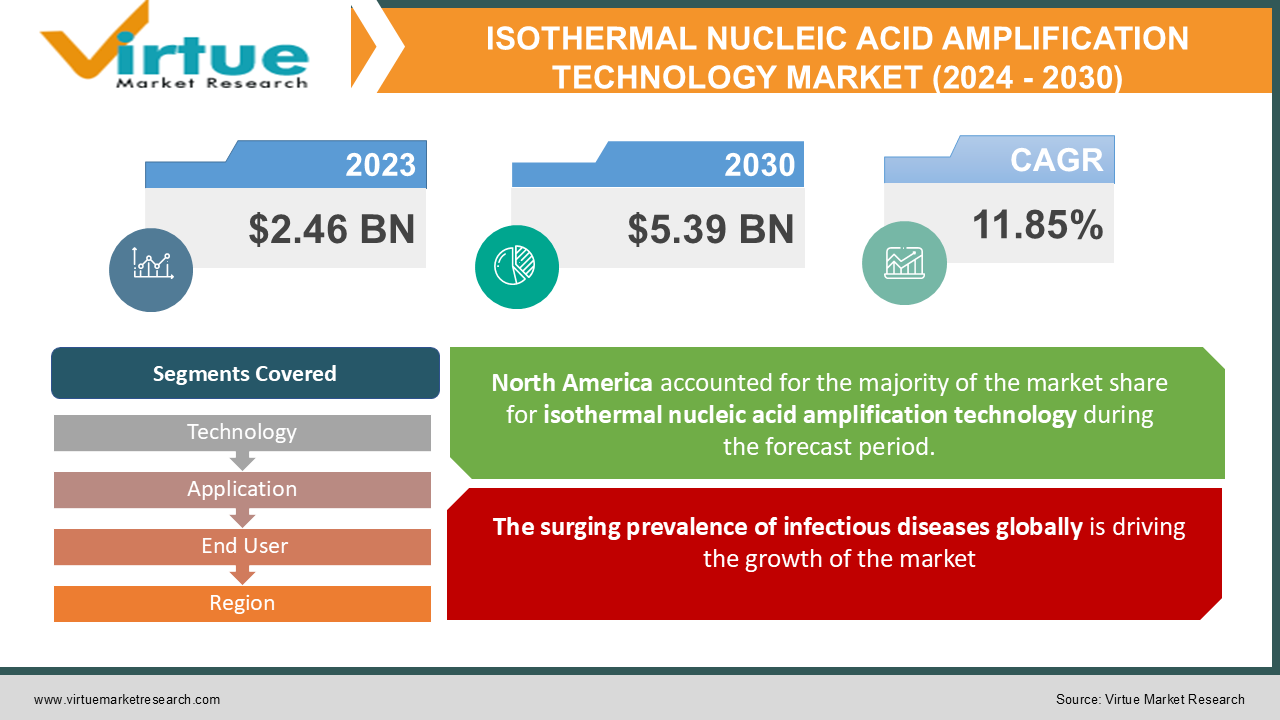
As per our research report, the global Isothermal Nucleic Acid Amplification Technology (INAAT) Market size was USD 2.46 billion in 2023 and is estimated to grow to USD 5.39 billion by 2030. This market is witnessing a healthy CAGR of 11.85% from 2024 - 2030. An increase in the number of infectious diseases and cancers ,the rise in the aging population across the globe and the increase in the adoption of INAAT over other molecular diagnostic techniques the growth of the industry.
Industry Overview:
Nucleic acid amplification technology is used in the field of molecular biology and recombinant DNA technology. These techniques are used as modern methods in detecting and analyzing small quantities of nucleic acids. Isothermal nucleic acid amplification processes are diverse and have many advantages, as they are extremely fast and do not require thermal cycling. INAAT is widely used in the United States, mainly to diagnose infectious diseases such as HIV/AIDS, H. influenzae, S. pneumonia (respiratory infection), N. gonorrhea, C. trachomatis (genital infection) and tuberculosis. According to the CDC, more than 2.8 million new chlamydia infections occur in the United States each year, and more than 2% of the young population already has it. Nucleic acid amplification has a number of applications in the healthcare industry, from research to the clinic. By far, PCR is the most widely used amplification technology in the world. A thermal circulator is necessary for PCR because different temperatures must be maintained at different stages of amplification. In addition, contaminants must be removed for effective amplification with this technology. The thermal circulator itself is expensive, and sample preparation to remove contaminants from the sample makes the overall process more costly and time-consuming.
Due to this high cost, this technique has had limited application in developing countries, where the demand for this technology is very high, especially for clinical applications. This high demand has led to the development of new innovative techniques, such as isothermal nucleic acid amplification technology. According to the World Health Organization (WHO), it is estimated that by 2020, chronic diseases will account for nearly three-quarters of all deaths worldwide. In addition, 71% of deaths will be due to ischemic heart disease (IC), 75% of deaths from stroke and 70% of deaths from diabetes will occur in developing countries.
COVID-19 impact on Isothermal Nucleic Acid Amplification Technology
The COVID19 pandemic has had a positive impact on the Isothermal Nucleic Acid Amplification Technology (INAAT) market. Since INAAT offers simple operation, quick detection, and accurate results. Therefore, it becomes ideal for primary testing centers in urban and rural areas. INAAT is in high demand because PCR-based nucleic acid detection technology is time-consuming and requires specialized equipment.
In addition, it is difficult to work with PCR in frontline medical facilities. Antibody-based detection has limitations because antibodies appear late, making early diagnosis of the virus difficult, while antigen-based detection has low sensitivity, which often leads to false-negative rates. high.
The isothermal nucleic acid amplification technology (INAAT) method enables rapid and exponential detection of target nucleic acid sequences. This method is not limited by thermal cycling constraints. Isothermal amplification, when integrated into a microsystem or mobile device, improves in situ nucleic acid-based diagnostics and increases sensitivity. Furthermore, by using different enzymatic approaches to separate the two strands of DNA prior to replication, INAAT was able to achieve amplification in 1516 min.
MARKET DRIVERS:
The surging prevalence of infectious diseases globally is driving the growth of the market
The rising rate of infectious diseases globally is expected to significantly drive the market expansion throughout the forecast period. An increase in the number of infectious diseases like flu, HIV, tuberculosis, etc. have increased the need for better diagnostic and treatment approaches in healthcare settings. According to the WHO's 2021 report, a total of 1.5 million people will die in 2020 from tuberculosis. Globally, TB is the 13th leading cause of death and the second leading cause of infectious death after COVID19 (before HIV/AIDS). Furthermore, by 2020, nearly 10 million people will be infected with TB worldwide. Including 5.6 million men, 3.3 million women, and 1.1 million children. Tuberculosis is present in all countries and of all ages.
Continues Technological Advancements are also driving the growth of the market
The usual choice for diagnosing these diseases is PCR. However, with advances in INAAT, diagnosis can be made quickly and cost-effectively. Therefore, the need to diagnose these diseases has increased the need for INAAT solutions. As a result, the proliferation of chronic diseases has driven the demand for INAAT diagnostic services thereby driving the overall growth of the Isothermal Nucleic Acid Amplification Technology market. Continuous improvements to existing amplification products as well as the introduction of new technologies such as Wire Shift Amplification (SDA), Single Primer Isotherm Amplification (SPIA), and Recombinant Polymerase Amplification (RPA) ) and regulatory change scenarios for these are expected to drive the market. The SMART2 technique is one of the great technological advancements.
MARKET RESTRAINTS:
High cost related to the test is restraining the growth of the market
Due to this high cost, this technique has had limited application in developing countries, where the demand for this technology is very high, especially for clinical applications. This high demand has led to the development of new innovative techniques, such as isothermal nucleic acid amplification technology. Keeping the test prices according to the people's demand and ability to purchase will help in the growth of the market.
GLOBAL ISOTHERMAL NUCLEIC ACID AMPLICATION TECHNOLOGY (INAAT) MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2022 - 2030 |
|
Base Year |
2022 |
|
Forecast Period |
2023 - 2030 |
|
CAGR |
11.85 % |
|
Segments Covered |
By Technology, Application, End User and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Alere, Inc., bioMerieux SA, Eiken Chemical Co. Ltd., Hologic, Inc. (Gen-Probe), Lucigen; QIAGEN, Quidel Corporation, Thermo Fisher Scientific, Becton, Dickinson & Company, Becton Dickinson and Company Qiagen NV, bioMerieux, |
This research report on the Isothermal Nucleic Acid Amplification Technology Market has been segmented and sub-segmented based on technology, application, delivery mode, end-user, and region.
Isothermal Nucleic Acid Amplification Technology Market - By Technology:
-
NASBA
-
HDA
-
LAMP
-
SDA
-
SPIA
-
NEAR
-
TMA
-
RCA
-
RPA
-
SMAP2
-
Others
Based on the Technology, Some of the technologies on the market include SPIA, SDA, Nucleic Acid Sequence Based Amplification (NASBA), Helicase Dependent Amplification (HDA), Loop Isothermal Amplification (LAMP), and Transcriptional Amplification (TMA). ). The global demand for INAAT has increased due to increasing customer awareness. TMA was the highest revenue-generating technology segment in the diagnostics market in 2016, as it was the first INAAT to be introduced to the market. It is developed by Hologic (Probe Gen).
Population growth with cancer and infectious diseases has fueled the need for low-cost diagnostic methods such as INAAT. Other amplification technologies such as polymerase chain reaction (PCR) cannot meet the diagnostic needs at a low cost. New technologies, including LAMP and HDA, are expected to show steady growth over the forecast period. Non-PCR technologies such as INAAT are gaining prominence due to the personalization features they provide.
Isothermal Nucleic Acid Amplification Technology Market - By Application:
-
Blood screening
-
Infectious disease diagnostics
-
Cancer
-
Others
Based on application, Isothermal amplification is mainly used to detect infectious diseases. This coupled with the advent of more specific kits, the increasing prevalence of diseases such as HIV, hepatitis A and B, and sexually transmitted diseases including chlamydia and gonorrhea are factors. is expected to drive the isothermal nucleic acid amplification technology market. In addition, the growing demand for rapid tests to detect infectious diseases to prevent their spread, especially in low-income countries, is expected to drive growth.
Blood screening is also an important application segment due to the increasing number of blood transfusions worldwide. INAAT is widely used to detect infectious organisms in transfused blood to limit their spread and prevent disease outbreaks. Several companies have performed extensive R&D to use INAAT in detecting cancer-specific molecular biomarkers and developing drugs specific for these biomarkers.
Isothermal Nucleic Acid Amplification Technology Market - By End-User:
-
Hospitals,
-
Central and reference labs
-
Others
Based on End-User, Hospitals dominated the segment with the largest market share in 2016. The development of wireless communication and miniaturized point-of-care INAAT devices facilitate hospital analysis by providing single-level access to diagnostic results throughout the patient institute. Automation, a large number of samples to detect infectious diseases, and the need for accurate results are the factors that are expected to strengthen the dominance.
According to end-user analysis, the reference laboratories segment is expected to show growth due to the increasing number of reference laboratories and the high adoption of new and major molecular diagnostic techniques. corpse. Various public funding agencies such as the CDC, and NIH, as well as private investors, such as the Wellcome Trust and the Bill & Melinda Gates Foundation, are driving the development of innovative technologies for rapid analysis of specific infectious diseases.
Isothermal Nucleic Acid Amplification Technology Market - By Region:
-
North America
-
Europe
-
Asia-Pacific
-
Latin America
-
The Middle East
-
Africa
Geographically, North America dominated the INAAT market segment in the region with the highest revenue in 2016. Economies in the region, including the United States and Canada, exhibited strong growth driven by their infrastructures. Research is established and research initiatives are evolving. The growth of the isothermal diagnostics market is driven by the increase in demand and investment from foreign companies, resulting in increasing competition.
Asia-Pacific is expected to be the fastest-growing region during the forecast period. China has experienced rapid urbanization due to strong economic growth. Increasing disposable income in developing economies, large populations, increased investment in health care facilities, and unmet demand for medical care are expected to promote growth. promote the adoption of INAAT devices. Demand for cost-effective diagnostic and preventive drugs is also expected to drive the market. The increasing prevalence of chronic diseases in the region will also have a positive effect on the market expansion. For example, according to the Centers for Disease Control and Prevention (CDC), in the United States in 2018, 1,708,921 new cases of cancer were reported and 599,265 people died of cancer. For every 100,000 people, 436 new cancer cases are reported and 149 people die from cancer. Furthermore, the increasing number of new product launches coupled with the high penetration of major market competitors in North America, drive the market further.
Isothermal Nucleic Acid Amplification Technology Market Share by company
-
Alere, Inc.
-
bioMerieux SA
-
Eiken Chemical Co. Ltd.
-
Hologic, Inc. (Gen-Probe)
-
Lucigen; QIAGEN
-
Quidel Corporation
-
Thermo Fisher Scientific
-
Becton
-
Dickinson & Company
-
Becton Dickinson and Company
-
Qiagen NV
-
bioMerieux,
Abbott announces that the U.S. Food and Drug Administration (FDA) has granted an Emergency Use Authorization (EUA) for the fastest available molecular test for the detection of novel coronavirus (COVID19), with positive results.. The test runs on the company's ID NOW platform, which provides rapid results in a range of healthcare settings such as doctor's offices, urgent care clinics and emergency department hospitals. This launch has helped to improve the company's product portfolio and strengthen its position in the market.
Thermo Fisher Scientific has come up with two new solutions based on Isothermal Reverse Transcriptional Amplification (RTLAMP). This nucleic acid-based RTLAMP isothermal amplification provides a quick and inexpensive option for the detection of viral pathogens. The introduction of this product will help the company expand its product portfolio, thereby boosting the market growth. Thermo Fisher Scientific has come up with two new solutions based on Isothermal Reverse Transcriptional Amplification (RTLAMP). This nucleic acid-based RT-LAMP isothermal amplification provides a quick and inexpensive option for the detection of viral pathogens. These product launches will help the company expand its product portfolio.
NOTABLE HAPPENINGS IN THE ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY MARKET IN THE RECENT PAST:
-
Product Launch - In March 2022, Thermo Fisher Scientific launched two new reverse transcriptions loop-mediated isothermal amplification (RT-LAMP)-based solutions. This isothermal RT-LAMP nucleic-acid-based amplification provides a rapid and low-cost option for viral pathogen detection.
- Authorization - In April 2020, Abbott announced that the U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the fastest available molecular point-of-care test for the detection of novel coronavirus (COVID-19), delivering positive results in as little as five minutes and negative results in 13 minutes.
Chapter 1. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY MARKET – Scope & Methodology
1.1. Market Segmentation
1.2. Assumptions
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY MARKET – Executive Summary
2.1. Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-19 Impact Analysis
2.3.1. Impact during 2024 - 2030
2.3.2. Impact on Supply – Demand
Chapter 3. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY MARKET – Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY MARKET - Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatory Scenario - By Region
4.3 Customer Analysis
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY MARKET - Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY MARKET – By Technology
6.1. NSBA
6.2. HAD
6.3. LAMP
6.4. SDA
6.5. SPIA
6.6. NEAR
6.7. TMA
6.8. RCA
6.9. RPA
6.10. SMAP2
6.11. Others
Chapter 7. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY MARKET – By Application
7.1. Blood Screening
7.2. Infectious disease diagnostics
7.3. Cancer
7.4. Others
Chapter 8. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY MARKET – By End – User
8.1. Hospitals
8.2. Central and Reference Labs
8.3. Others
Chapter 9. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY MARKET - By Region
9.1. North America
9.2. Europe
9.3. Asia-P2acific
9.4. Latin America
9.5. The Middle East
9.6. Africa
Chapter 10. ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY MARKET – By Companies
10.1. Alere Inc.
10.2. bioMerieux SA
10.3. Eiken Chemical Co. Ltd.
10.4. Hologic, Inc.
10.5. Lucigen
10.6. QIAGEN
10.7. Quidel Corporation
10.8. Thermo Fisher Scientific
10.9. Becton
10.10. Dickinson & Company
10.11. Becton Dickinson and Company
10.12. Qiagen NV
10.13. bioMerieux
Download Sample
Choose License Type
2500
4250
5250
6900