Gene Therapy Market Size (2025 – 2030)
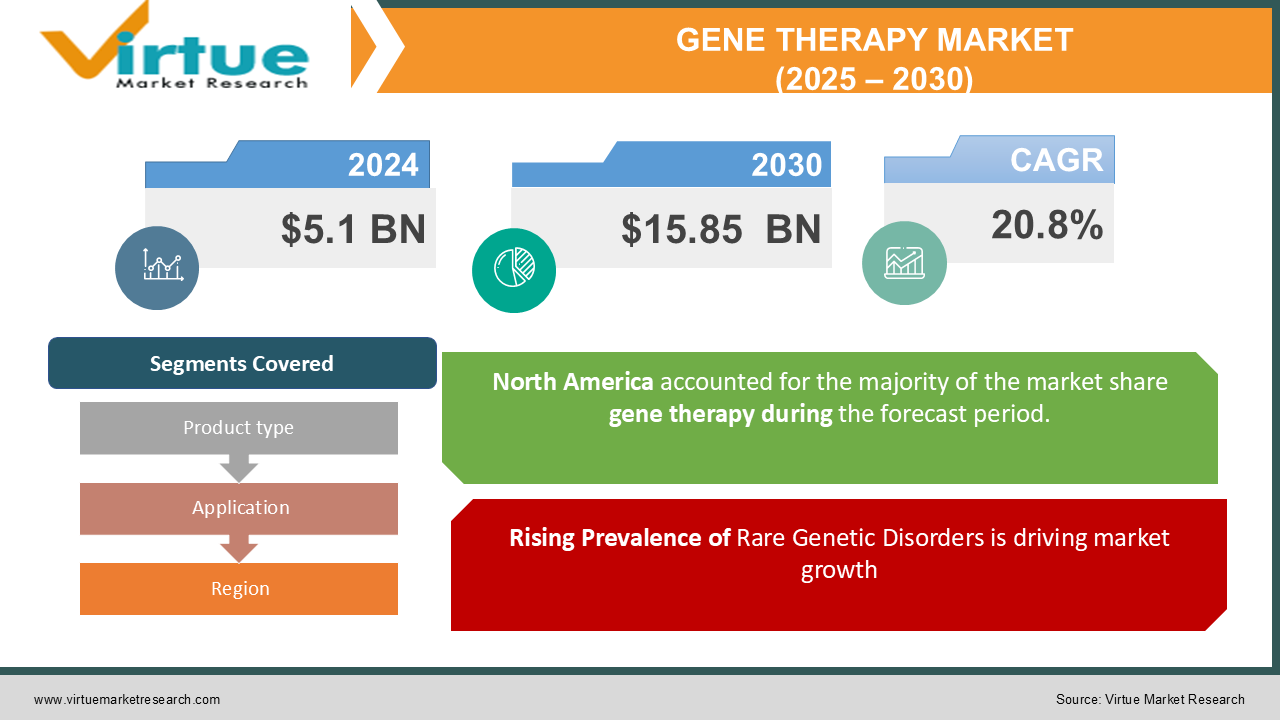
The Global Gene Therapy Market was valued at USD 5.1 billion in 2024 and is projected to grow at a CAGR of 20.8% from 2025 to 2030. By 2030, the market is expected to reach USD 15.85 billion.
Gene therapy focuses on the delivery of genetic material into cells to replace faulty genes, introduce new genes, or silence harmful genes to treat or prevent diseases. This market is poised for exponential growth, driven by advancements in gene-editing technologies such as CRISPR, increasing FDA approvals for gene therapies, and rising investments in biotechnology. With applications spanning rare genetic disorders, oncology, and cardiovascular diseases, gene therapy offers transformative potential in modern medicine.
Key Market Insights:
-
Gene therapy is gaining traction for rare genetic disorders, with several recent FDA-approved treatments targeting conditions like spinal muscular atrophy (SMA) and inherited retinal diseases.
-
Oncology represents one of the fastest-growing application segments, with gene therapies being developed to treat various forms of cancer, including leukemia and melanoma. Advances in genome editing tools, particularly CRISPR-Cas9 and TALENs, are streamlining the discovery and application of gene therapies.
-
Increased government funding and private investments in biotechnology have bolstered research and development, with significant contributions from regions like North America and Europe.
-
Ethical and regulatory challenges continue to pose hurdles, with concerns surrounding off-target effects, long-term efficacy, and equitable access to treatments.
-
Expensive manufacturing processes and logistical complexities in delivering gene therapies remain significant barriers, particularly in developing markets.
-
The rapid evolution of delivery technologies, including non-viral vectors such as nanoparticles and lipid-based carriers, is reducing dependency on viral systems.
Global Gene Therapy Market Drivers:
Rising Prevalence of Rare Genetic Disorders is driving market growth:
Gene therapy has emerged as a groundbreaking treatment option for rare genetic disorders, many of which were previously considered untreatable. Conditions such as Duchenne muscular dystrophy (DMD), cystic fibrosis, and spinal muscular atrophy (SMA) are receiving innovative treatment solutions through gene therapies. Traditional therapies often manage symptoms without addressing the root causes, while gene therapy offers the potential for a one-time curative approach. This transformative promise, coupled with growing awareness and diagnostic capabilities for rare diseases, is driving demand. Additionally, initiatives such as orphan drug designations and government incentives for rare disease research are fueling investment in this sector.
Advancements in Gene-Editing Technologies is driving market growth:
The advent of precise gene-editing tools like CRISPR-Cas9 has revolutionized the field of gene therapy by enabling accurate modifications of genetic sequences. These tools have significantly reduced the time and cost required for developing gene therapies, accelerating their application across diverse indications. CRISPR technology is being leveraged to correct genetic mutations, knock out disease-causing genes, and introduce protective genetic variations. Its adaptability and efficiency have unlocked possibilities for treating complex diseases, including certain types of cancer and neurodegenerative disorders, further strengthening its role as a key market driver.
Expanding Pipeline of Gene Therapies is driving market growth:
Pharmaceutical and biotech companies are investing heavily in the research and development of gene therapies, leading to a robust pipeline of treatments. Several gene therapies targeting various indications, including hemophilia, sickle cell disease, and age-related macular degeneration, are in late-stage clinical trials. The continuous approval of breakthrough therapies and their subsequent commercialization are expected to propel market growth. Regulatory authorities such as the FDA and EMA have expedited the review processes for gene therapy candidates, fostering a favorable environment for innovation and adoption.
Global Gene Therapy Market Challenges and Restraints:
High Cost of Treatment is restricting market growth:
Gene therapies are among the most expensive medical treatments globally, with some therapies costing millions per patient. The high cost is attributed to complex manufacturing processes, stringent quality control measures, and the need for personalized treatment approaches. For instance, Zolgensma, a gene therapy for SMA, is priced at over USD 2 million per patient. Such exorbitant costs limit accessibility, particularly in low- and middle-income countries. Insufficient insurance coverage and funding mechanisms exacerbate the affordability challenge, hindering the widespread adoption of gene therapy.
Regulatory and Ethical Barriers is restricting market growth:
The gene therapy market faces significant regulatory and ethical challenges, particularly concerning safety and long-term efficacy. Off-target effects, immune responses to viral vectors, and potential unintended genetic modifications raise concerns about patient safety. Additionally, ethical debates around germline editing and the potential misuse of gene-editing technologies complicate the regulatory landscape. Striking a balance between fostering innovation and ensuring ethical compliance is a persistent challenge for governments and industry stakeholders alike.
Market Opportunities:
The gene therapy market presents immense opportunities as scientific advancements continue to push the boundaries of medical innovation. Emerging economies, particularly in Asia-Pacific and Latin America, represent untapped markets with growing healthcare infrastructures and increasing investments in biotechnology. Expanding applications in oncology offer another significant growth avenue. Gene therapies such as CAR-T cell therapies have shown remarkable efficacy in treating hematologic malignancies, and their potential use in solid tumors is under active investigation. This evolving scope highlights the opportunity for biopharma companies to diversify their portfolios. Furthermore, the development of scalable manufacturing techniques and advanced delivery systems is expected to reduce production costs and enhance accessibility. Partnerships between academic institutions, pharmaceutical companies, and government agencies can further expedite the development and deployment of gene therapies globally.
GENE THERAPY MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
20.8% |
|
Segments Covered |
By Product type, Application, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Novartis AG, Spark Therapeutics, Inc., Bluebird Bio, Inc., Sarepta Therapeutics, Inc., Orchard Therapeutics plc, Regenxbio Inc., UniQure N.V., Biomarin Pharmaceutical Inc., Pfizer Inc., Gilead Sciences, Inc. |
Gene Therapy Market Segmentation: By Product Type
-
Viral Vectors
-
Non-Viral Vectors
Viral vectors dominate the market due to their efficiency and reliability in delivering therapeutic genes. Adeno-associated viruses (AAV) are particularly favored for their low immunogenicity and ability to target diverse cell types.
Gene Therapy Market Segmentation: By Application
-
Oncology
-
Rare Genetic Disorders
-
Cardiovascular Diseases
-
Neurological Disorders
-
Others
Oncology is the leading application segment, with the development of advanced therapies such as CAR-T cells and oncolytic viruses driving growth. These therapies have demonstrated exceptional efficacy in treating cancers, particularly hematologic malignancies.
Gene Therapy Market Segmentation: By Region
-
North America
-
Europe
-
Asia-Pacific
-
South America
-
Middle East and Africa
North America is the dominant region in the global gene therapy market, accounting for the largest share due to advanced healthcare infrastructure, high R&D investments, and a favorable regulatory environment. The presence of leading biotech companies and a strong clinical pipeline further reinforce the region's position. The U.S., in particular, leads the market with numerous FDA-approved therapies and a robust ecosystem supporting biotech innovation. The government’s emphasis on accelerating precision medicine initiatives continues to foster growth in this region.
COVID-19 Impact Analysis on the Gene Therapy Market:
The COVID-19 pandemic had a dual impact on the gene therapy market. On one hand, many clinical trials were delayed due to the reallocation of resources to COVID-19 research and disruptions in logistics and healthcare services. These delays hindered the progress of some gene therapies, slowing down their development timelines. However, on the other hand, the pandemic highlighted the crucial role of genetic medicine in addressing viral diseases, such as COVID-19. The success of mRNA vaccines, which are based on gene technology, underscored the potential of genetic interventions and boosted both public and investor confidence in the field of gene therapy. The pandemic also acted as a catalyst for innovation in the gene therapy space. It accelerated the development of advanced gene delivery systems and manufacturing techniques, improving the efficiency and scalability of gene therapy production. These innovations are laying the groundwork for future growth in the sector, particularly in the areas of viral vector development and CRISPR gene editing technologies. Post-pandemic, the gene therapy market is poised for rapid recovery and growth. As the healthcare system stabilizes, clinical trials are resuming, and many gene therapies are moving closer to regulatory approval. The urgency of pandemic-driven medical breakthroughs has increased global focus on healthcare innovation, providing a favorable environment for gene therapy advancements. This renewed interest, coupled with continued improvements in gene therapy techniques and increased investment, positions the market for significant expansion in the coming years. Overall, the pandemic not only delayed progress but also helped to cement the role of genetic medicine in the future of healthcare, accelerating the gene therapy market’s evolution and setting the stage for an era of groundbreaking treatments.
Latest Trends/Developments:
The gene therapy market is undergoing significant transformations, driven by several key trends. One of the most notable advancements is the rise of non-viral delivery systems, such as lipid nanoparticles and exosome-based carriers. These innovative methods offer safer and more efficient alternatives to traditional viral vectors, reducing potential risks and improving the delivery of genetic material to target cells. Another major development is the application of CRISPR-Cas9 technology for in vivo gene editing. This breakthrough allows for direct genetic corrections within the human body, offering new hope for treating genetic disorders such as sickle cell disease and beta-thalassemia. Early results in these areas have shown promising potential, bringing gene therapy closer to becoming a viable treatment for previously untreatable conditions. In addition, advancements in Chimeric Antigen Receptor T-cell (CAR-T) therapies are expanding their use beyond hematologic cancers, opening up new possibilities for treating solid tumors. This evolution of CAR-T technology is pushing the boundaries of immunotherapy and enhancing its therapeutic potential for a broader range of cancers. The integration of artificial intelligence (AI) and machine learning (ML) is further accelerating progress in gene therapy. These technologies are being leveraged to enhance the speed and accuracy of candidate identification, helping researchers pinpoint the most promising gene therapies more efficiently. AI and ML are also improving the design of clinical trials, optimizing treatment regimens, and predicting patient outcomes with greater precision. Together, these trends are driving the gene therapy market toward a future of more effective, accessible, and personalized treatments, positioning the field for continued innovation and expansion in the coming years.
Key Players:
-
Novartis AG
-
Spark Therapeutics, Inc.
-
Bluebird Bio, Inc.
-
Sarepta Therapeutics, Inc.
-
Orchard Therapeutics plc
-
Regenxbio Inc.
-
UniQure N.V.
-
Biomarin Pharmaceutical Inc.
-
Pfizer Inc.
-
Gilead Sciences, Inc.
Chapter 1. Gene Therapy Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Gene Therapy Market – Executive Summary
2.1 Market Size & Forecast – (2025 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Gene Therapy Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Gene Therapy Market - Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Gene Therapy Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Gene Therapy Market – By Product Type
6.1 Introduction/Key Findings
6.2 Viral Vectors
6.3 Non-Viral Vectors
6.4 Y-O-Y Growth trend Analysis By Product Type
6.5 Absolute $ Opportunity Analysis By Product Type, 2025-2030
Chapter 7. Gene Therapy Market – By Application
7.1 Introduction/Key Findings
7.2 Oncology
7.3 Rare Genetic Disorders
7.4 Cardiovascular Diseases
7.5 Neurological Disorders
7.6 Others
7.7 Y-O-Y Growth trend Analysis By Application
7.8 Absolute $ Opportunity Analysis By Application, 2025-2030
Chapter 8. Gene Therapy Market , By Geography – Market Size, Forecast, Trends & Insights
8.1 North America
8.1.1 By Country
8.1.1.1 U.S.A.
8.1.1.2 Canada
8.1.1.3 Mexico
8.1.2 By Product Type
8.1.3 By Application
8.1.4 Countries & Segments - Market Attractiveness Analysis
8.2 Europe
8.2.1 By Country
8.2.1.1 U.K
8.2.1.2 Germany
8.2.1.3 France
8.2.1.4 Italy
8.2.1.5 Spain
8.2.1.6 Rest of Europe
8.2.2 By Product Type
8.2.3 By Application
8.2.4 Countries & Segments - Market Attractiveness Analysis
8.3 Asia Pacific
8.3.1 By Country
8.3.1.1 China
8.3.1.2 Japan
8.3.1.3 South Korea
8.3.1.4 India
8.3.1.5 Australia & New Zealand
8.3.1.6 Rest of Asia-Pacific
8.3.2 By Product Type
8.3.3 By Application
8.3.4 Countries & Segments - Market Attractiveness Analysis
8.4 South America
8.4.1 By Country
8.4.1.1 Brazil
8.4.1.2 Argentina
8.4.1.3 Colombia
8.4.1.4 Chile
8.4.1.5 Rest of South America
8.4.2 By Product Type
8.4.3 By Application
8.4.4 Countries & Segments - Market Attractiveness Analysis
8.5 Middle East & Africa
8.5.1 By Country
8.5.1.1 United Arab Emirates (UAE)
8.5.1.2 Saudi Arabia
8.5.1.3 Qatar
8.5.1.4 Israel
8.5.1.5 South Africa
8.5.1.6 Nigeria
8.5.1.7 Kenya
8.5.1.8 Egypt
8.5.1.9 Rest of MEA
8.5.2 By Product Type
8.5.3 By Application
8.5.4 Countries & Segments - Market Attractiveness Analysis
Chapter 9. Gene Therapy Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
9.1 Novartis AG
9.2 Spark Therapeutics, Inc.
9.3 Bluebird Bio, Inc.
9.4 Sarepta Therapeutics, Inc.
9.5 Orchard Therapeutics plc
9.6 Regenxbio Inc.
9.7 UniQure N.V.
9.8 Biomarin Pharmaceutical Inc.
9.9 Pfizer Inc.
9.10 Gilead Sciences, Inc.
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The Global Gene Therapy Market was valued at USD 5.1 billion in 2024 and is projected to grow at a CAGR of 20.8% from 2025 to 2030. By 2030, the market is expected to reach USD 15.85 billion.
Key drivers include the rising prevalence of rare genetic disorders, advancements in gene-editing technologies, and an expanding pipeline of gene therapy candidates.
The market is segmented by vector type (viral and non-viral vectors) and by application (oncology, rare genetic disorders, cardiovascular diseases, etc.).
North America dominates the market due to advanced healthcare infrastructure, significant R&D investments, and a favorable regulatory framework.
Leading players include Novartis AG, Spark Therapeutics, Inc., Bluebird Bio, Inc., Sarepta Therapeutics, Inc., and Orchard Therapeutics plc.