External VNS Devices Market Size (2024 - 2030)
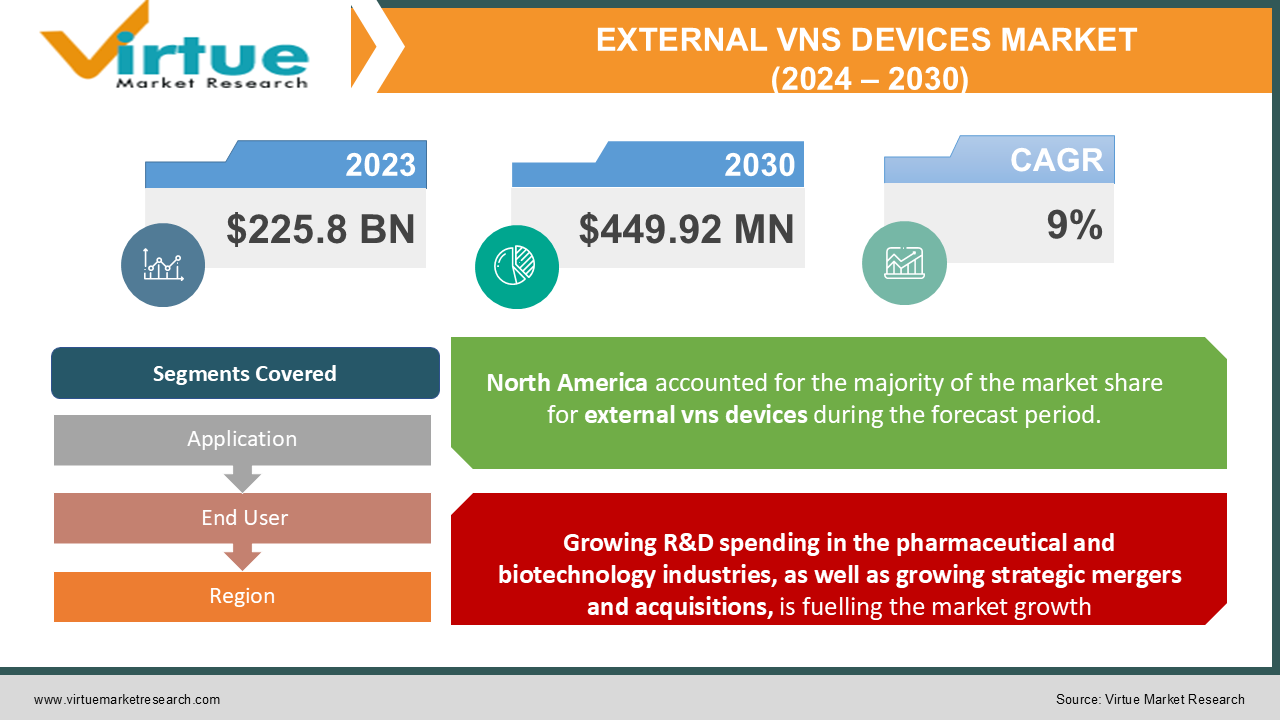
In 2023, the Global External VNS Devices Market was valued at $225.8 billion, and is projected to reach a market size of $449.92 Million by 2030. Over the forecast period of 2024-2030, market is projected to grow at a CAGR of 9%.
Industry Overview:
Vagus nerve stimulation (VNS) therapy aims to prevent seizures by delivering regular, gentle electrical pulses to the brain via the vagus nerve. A device that resembles a pacemaker is responsible for delivering these pulses. The autonomic nervous system, which regulates unconscious bodily processes including heart rate, is made up of the vagus nerve. To reach the lower portion of the brain, it flows through the neck, which is located between the chest and abdomen. The VNS device, sometimes known as the "pacemaker of the brain," is positioned beneath the skin on the chest wall. It has a cable that connects to the vagus nerve in the neck. Intractable epilepsy and depression that is resistant to treatment are treated using vagus nerve stimulators. The external Vagal Nerve Stimulation (VNS) devices rely solely on electrical signals that can pass unaffected through the skin and muscles of the neck. This process must be exact enough to control and access particular neuronal activity. The vagal nerve stimulator market is anticipated to increase as a result of advances in technology and the release of new external VNS devices.
The prevalence of neurological conditions including migraine and epilepsy is rising across all age groups, and the adoption of this technology has produced favorable clinical results, which are the main drivers driving the growth of the worldwide vagus nerve stimulation market. Neurological illnesses are becoming more prevalent in emerging economies throughout time. For the previous few decades, vagus nerve stimulator therapy has been used to treat a variety of difficult conditions, including treating neurological tremors and dyskinesia. This mechanism is capable of causing muscle contractions and regaining motor nerve functioning. In addition, vagus nerve stimulators can be utilized by patients to regain mobility, bladder control, and hand grasp. With the aid of a vagus nerve stimulator, additional neurological conditions such as migraines, Crohn's disease, asthma, depression, headaches, and cardiovascular problems can be brought back to normal. However, a lack of qualified workers and the expensive price of neurostimulator devices limit market expansion. In contrast, it is anticipated that increased R&D efforts to broaden the indications for the use of neurostimulators will offer attractive market expansion potential.
COVID-19 pandemic impact on External VNS Devices Market
Vagal nerve stimulation device manufacture is significantly impacted by COVID-19. Inventory management, production lines, workforce, and supply chains in the market have been disrupted by the pandemic and effective lockdowns imposed by nations including the United States, China, India, Japan, Germany, the United Kingdom, and others. Furthermore, because of the disruption in their raw material sources, the supply chain networks have been severely impacted. The production of the VNS device during the COVID-19 pandemic phase was significantly impacted by the pandemic's remote functioning. Manufacturers have been able to alter the pricing structure thanks to the losses brought on by the pandemic. Each product's pricing scheme is influenced by its production costs, R&D expenditures, supply chain costs, and inventory costs. Nevertheless, the COVID-19 pandemic is anticipated to have a positive effect on the market for vagus nerve stimulators due to the association between COVID-19 and neurological disorders like depression and anxiety as well as the growing research into the possibility of using vagus nerve stimulators as a treatment or management for COVID-19.
MARKET DRIVERS:
The global external vagus nerve stimulators market is anticipated to rise as a result of the rising prevalence of neurological disorders like epilepsy, depression, anxiety, and others
Depression is a widespread mental condition, according to data released by the World Health Organization (WHO). Due to this rising prevalence, there is a rising demand for a variety of tools and treatments, such as vagal nerve stimulation (VNS). More neurological problems are being treated with vagal nerve stimulation. An increasing variety of neurological conditions are being treated with vagal nerve stimulation. The external vagus nerve stimulator does not require implantation, hence it is anticipated to offer a high level of hygienic and non-infectious profile. In addition, patients may afford the external VNS devices and they are more successful in terms of treatment.
Growing R&D spending in the pharmaceutical and biotechnology industries, as well as growing strategic mergers and acquisitions, is fuelling the market growth
The rapid use of vagal nerve stimulation has been made possible by the rise in neurological illnesses. Additionally, more research and development have resulted in the introduction of new, technologically advanced products to the market. Start-up businesses are receiving enormous financial support as they create competitive goods for vagal nerve stimulation treatments and surgery. Furthermore, it is anticipated that the growing number of vagal nerve stimulation patents from the new start-up firms would allow them to contribute to a significant market share in the vagal nerve stimulation market. Due to their highly skilled workforces and cutting-edge products, these up-and-coming start-up businesses provide considerable strategic mergers and acquisition chances for the major market players. As a result, it is anticipated that over the projected period, the market for vagal nerve stimulation devices is likely to be benefitted from the rising strategic mergers and acquisitions.
MARKET RESTRAINTS:
An increase in the cost of vagal nerve stimulation therapy may restrain the market growth
The global use of VNS devices and therapies is being fueled by the rising incidences of numerous neurological illnesses, including depression, epilepsy, and other conditions. Additionally, there are not many patients receiving therapy for these neurological illnesses, which has had a significant impact on the pricing structure of the VNS procedure because many people still favor receiving medical attention for these neurological problems. The uptake of VNS devices and treatments is significantly impacted by this aspect. Additionally, the expensive expense of VNS surgery is having a big impact on the uptake of VNS devices. In conclusion, the vagal nerve stimulation market is anticipated to have slow development throughout the projected period due to the high cost of VNS therapy, high surgical costs, and popular medical therapies.
The market for vagus nerve stimulators is likely to be constrained by risks associated with technical failures and high costs
The use of vagus nerve stimulators has a wide range of potential side effects. Other concurrent technical issues include generator and dislocation malfunction. For instance, a June 2018 article in the monthly peer-reviewed medical journal World Neurosurgery stated that some difficulties with vagus nerve stimulators can be brought on by lead breakage, disconnection, battery displacement, or device malfunction. Late-stage consequences include laryngopharyngeal dysfunction, which affects roughly 66% of patients with implanted vagus nerve stimulators. Early stage issues include microbiological infections, dyspnea (shortness of breath), etc.
EXTERNAL VNS DEVICES MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
9% |
|
Segments Covered |
By Application, End User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Medtronic Plc., Nevro Corporation, ElectroCore LLC, Boston Scientific Corporation, LivaNova Plc., BioControl Medical, Bioness Inc., Aleva Neurotherapeutics SA, EnteroMedics Inc., and NeuroPace Inc., ReShape Lifesciences, Inc., and NERVANA LLC, |
This research report on the External VNS Devices market has been segmented based on application, end user, and region.
External VNS Devices Market– By Application
- Depression
- Epilepsy
- Others
Based on Application, the External VNS Devices Market is bifurcated into Depression, Epilepsy, and Others. Depression is treatable with a correct diagnosis, counseling, and medication. With the use of stimulation therapies like nerve stimulation devices, depression can now be efficiently treated thanks to technological breakthroughs in the healthcare industry. The neurological condition of epilepsy is characterized by aberrant brain activity that results in seizures or moments of strange behavior, loss of awareness, and sensations. For those who have epilepsy, treatment with drugs or surgery can manage seizures. In addition to the uses listed above, vagal nerve stimulation devices can be used to treat or cure a wide range of neurological conditions.
External VNS Devices Market– By End User
- Hospitals
- Ambulatory Surgical Centers
- Others
Based on End User, the External VNS Devices Market is bifurcated into Hospitals, Ambulatory Surgical Centers, and Others. The market for vagal nerve stimulation is anticipated to be driven by the rise of multispecialty clinics for neurology, cardiology, and other essential disorders in developed nations. In hospitals throughout the world, the vagal nerve stimulator is frequently used to treat epilepsy that is uncontrolled by conventional therapy. Nerve stimulation therapy reduces the frequency of seizures by gently electrically stimulating the brain via the vagal nerve. The patient recovers more quickly after VNS therapy, has less severe seizures, and has a better quality of life and attentiveness. Ambulatory surgical centers are the places where procedures that don't call for hospital admission are carried out. Compared to many hospitals, ambulatory surgery centers offer a more comfortable setting and cost-effective procedures. Patients who choose to undergo surgery at an ambulatory surgical center arrive on the day of their procedure, have their surgery in a fully furnished operating room and recover while being attended to by trained nurses all without having to check into a hospital. The vagal nerve stimulation devices are employed for several research projects by government research institutes and commercial companies, in addition to the end-user sectors mentioned above. These businesses concentrate on enhancing services by innovating the current product line. In the upcoming years, it is anticipated that the market would expand in part due to the growth of healthcare facilities in important developing nations.
External VNS Devices Market - By Region:
- North America
- Europe
- Asia-Pacific
- Rest of the World
North America is anticipated to display a CAGR of 9.87% during the projection period. Due to its advanced technology, rising healthcare costs, expanding prevalence of neurological illnesses, and growing government support for R&D, North America is predicted to dominate the market for external vagal nerve stimulation devices. Additionally, the market in this region has grown due to rising R&D activity and the presence of significant corporations. Europe comes second in the global market for external vagal nerve stimulation devices. This is a result of the significant government funding and support for research and development. The Asia-Pacific external vagal nerve stimulation devices market is anticipated to grow at the second-highest with a CAGR of 10.69% over the projected period. Due to its vast patient population, rapidly evolving healthcare technology, and high healthcare spending, Asia-Pacific has the second-fastest expanding vagal nerve stimulation market after Europe. Additionally, expanding chances in nations like India, Japan, China, and South Korea will contribute to this market's global expansion. Latin American countries are anticipated to have substantial growth possibilities Due to the increase in healthcare infrastructure in this region's developing nations.
Major Key Players in the Market
The major players in the global External VNS Devices Market are
- Medtronic Plc.
- Nevro Corporation
- ElectroCore LLC
- Boston Scientific Corporation
- LivaNova Plc.
- BioControl Medical
- Bioness Inc.
- Aleva Neurotherapeutics SA
- EnteroMedics Inc.
- NeuroPace Inc.
- ReShape Lifesciences, Inc.,
- NERVANA LLC
Notable happenings in the External VNS Devices Market in the recent past:
- Product Approval- In April 2020, The American business electroCore, Inc., which develops medical technology, stated that the U.S. Food and Drug Administration had approved an additional indication for its non-invasive vagus nerve stimulator gammaCore, which now includes the prevention of migraine in adult patients. GammaCore was previously recommended as a complementary therapy for cluster headaches as well as for pain control in cases of episodic cluster headaches and migraine headaches.
Chapter 1.EXTERNAL VNS DEVICES MARKET – Scope & Methodology
1.1. Market Segmentation
1.2. Assumptions
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2.EXTERNAL VNS DEVICES MARKET – Executive Summary
2.1. Market Size & Forecast – (2022 – 2026) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-16 Impact Analysis
2.3.1. Impact during 2022 - 2026
2.3.2. Impact on Supply – Demand
Chapter 3.EXTERNAL VNS DEVICES MARKET – Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4.EXTERNAL VNS DEVICES MARKET - Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatory Scenario - By Region
4.3 Customer Analysis
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. EXTERNAL VNS DEVICES MARKET - Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6.EXTERNAL VNS DEVICES MARKET – By Application
6.1. Depression
6.2. Epilepsy
6.3. Others
Chapter 7.EXTERNAL VNS DEVICES MARKET – By End-User
7.1. Hospitals
7.2. Ambulatory Surgical Centers
7.3. Others
Chapter 8.EXTERNAL VNS DEVICES MARKET – By Region
8.1. North America
8.2. Europe
8.3. The Asia Pacific
8.4. Latin America
8.5. The Middle East
8.6. Africa
Chapter 9.EXTERNAL VNS DEVICES MARKET – Company Profiles – (Overview, Product Portfolio, Financials, Developments)
9.1. Medtronic Plc
9.2. Nevro Corporation
9.3. ElectroCore LLC
9.4. Boston Scientific Corporation.
9.5. LivaNova Plc
9.6. BioControl Medical
9.7. Bioness Inc
9.8. Aleva Neurotherapeutics SA
9.9. EnteroMedics Inc.
9.10. NeuroPace Inc
9.11. ReShape Lifesciences
9.12. NERVANA LLC
Download Sample
Choose License Type
2500
4250
5250
6900



