Endometrial Carcinoma Testing By Endometrial Biopsy Market Size (2024 – 2030)
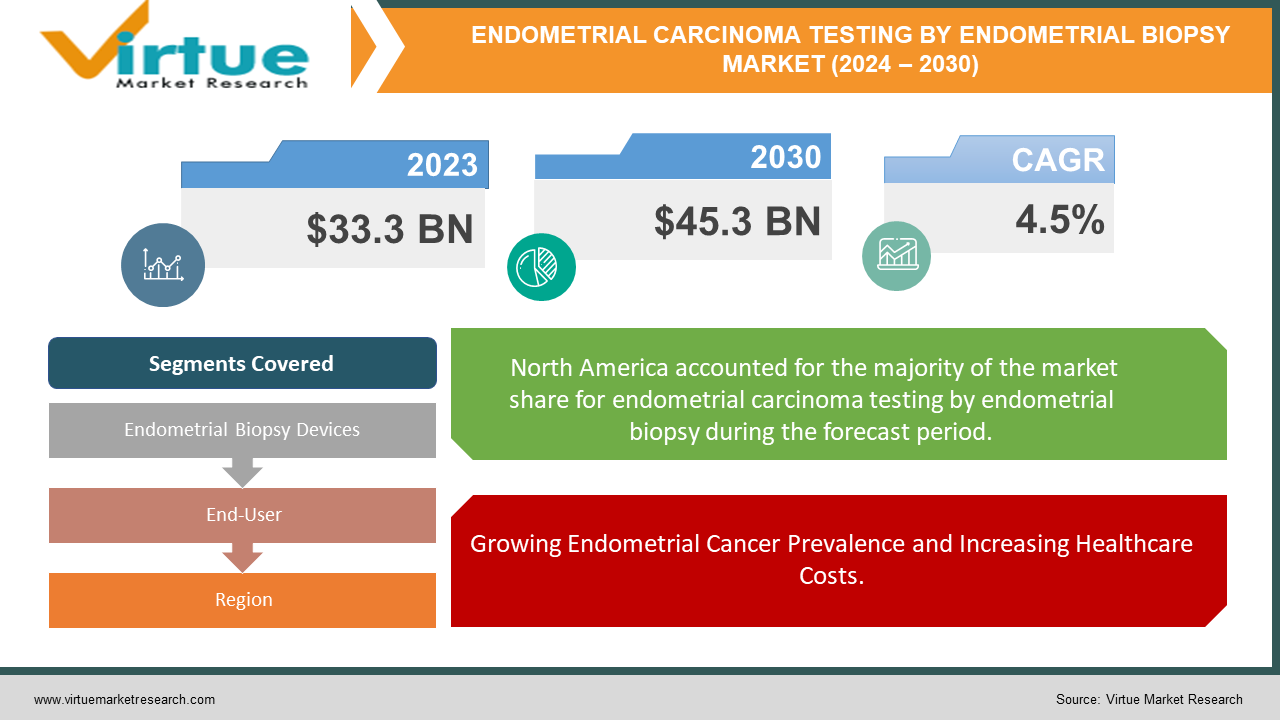
The Global Endometrial Carcinoma Testing By Endometrial Biopsy Market was valued at USD 33.3 billion in 2023 and is projected to reach a market size of USD 45.3 billion by the end of 2030. The market is anticipated to expand at a compound annual growth rate (CAGR) of 4.5% between 2024 and 2030.
The global market for endometrial carcinoma testing through endometrial biopsy serves as a pivotal tool in diagnosing uterine cancer, focusing specifically on definitive diagnoses through tissue sampling. Projected significant growth in this segment is driven by increasing awareness of early cancer detection and the popularity of minimally invasive procedures. However, challenges such as patient discomfort with traditional biopsy methods and the absence of a universal screening test persist. Yet, advancements in biopsy techniques offer promise in overcoming these limitations, alongside the potential of liquid biopsies to reshape the market landscape. As technology evolves, stakeholders must remain adaptable, capitalizing on emerging opportunities and addressing evolving challenges to drive continued growth in the sector.
Key Market Insights:
An increase in disposable endometrial biopsy catheters' market share is expected and 20-30% growth is projected over the next five years.
The Asia Pacific region is anticipated to witness the fastest market growth. This is driven by rising endometrial cancer prevalence and increasing disposable income.
North America dominates holding 40% of the global endometrial cancer market share in 2023 due to high healthcare expenditure and established infrastructure.
Specialized gynecological clinics serve niche markets. Estimated to cover 5-10% of the endometrial biopsy market, offering convenience and focused care.
Advancements in minimally invasive techniques and technology lead to thinner catheters and local anesthesia techniques, reducing patient discomfort during biopsies.
Global Endometrial Carcinoma Testing By Endometrial Biopsy Market Drivers:
Growing Endometrial Cancer Prevalence and Increasing Healthcare Costs.
The alarming increase in endometrial cancer cases is driving the global market for endometrial biopsy-based endometrial carcinoma testing. Owing to lifestyle variables like obesity and increased usage of hormone replacement medication, developed nations such as North America and Europe have higher incidence rates. There is an increasing need for precise and conclusive diagnostic instruments due to the expanding patient pool. Given its well-established effectiveness, endometrial biopsies become essential for either ruling out or verifying endometrial cancer. Additionally, the global increase in healthcare spending fosters a favorable climate for this industry. Developed nations with strong healthcare systems are more likely to spend money on cutting-edge diagnostic tools and treatments like endometrial biopsies. This financial support makes it easier for these techniques to be adopted more widely.
Growing Awareness About Female Health and Early Cancer Detection.
The global endometrial carcinoma testing by endometrial biopsy market thrives on a growing wave of awareness about female health and the importance of early cancer detection. Public health campaigns, patient advocacy groups, and media outreach have all contributed to women becoming more informed about risk factors, symptoms, and screening options for endometrial cancer. This heightened awareness empowers women to seek medical attention promptly upon experiencing concerning symptoms, like abnormal uterine bleeding. Early diagnosis is crucial for improving treatment outcomes and patient survival rates. Consequently, this increased awareness translates into a greater demand for diagnostic procedures like endometrial biopsy. As women become more proactive about their health, they're likely to discuss their concerns with healthcare providers and opt for recommended screening tests, including endometrial biopsy if necessary. This shift in patient behavior positively impacts the market for endometrial carcinoma testing by endometrial biopsy.
Global Endometrial Carcinoma Testing By Endometrial Biopsy Market Restraints and Challenges:
The global market for endometrial carcinoma testing through endometrial biopsy encounters several significant challenges that impact its effectiveness and accessibility. Foremost among these challenges is the absence of a universally accepted screening test, leading to reliance on symptomatic presentation for diagnosis and potentially limiting the market reach due to fewer preventive biopsies. Moreover, the discomfort and potential complications associated with the biopsy procedure deter some individuals, further impeding market growth. Additionally, while considered the gold standard, the biopsy's accuracy can be compromised by sampling errors or subjective interpretation, highlighting the need for advancements in technique and analysis. Cost considerations also pose a barrier, particularly in regions with limited healthcare access. Addressing these challenges requires ongoing research and development to enhance biopsy techniques, improve accuracy, and make the procedure more accessible, ultimately facilitating early detection and effective management of endometrial cancer.
Global Endometrial Carcinoma Testing By Endometrial Biopsy Market Opportunities:
The global market for endometrial carcinoma testing through endometrial biopsy is poised for dynamic growth, propelled by innovative advancements and evolving healthcare paradigms. Continuous research into minimally invasive biopsy techniques promises to enhance patient comfort and encourage broader adoption of the procedure, facilitating earlier diagnoses and expanding the market's potential. Furthermore, the development of liquid biopsies offers a revolutionary non-invasive approach to screening, potentially transforming endometrial cancer diagnosis by reaching a wider demographic, including asymptomatic individuals. The growing emphasis on personalized medicine further underscores the importance of endometrial biopsy procedures, as molecular testing becomes increasingly integral in tailoring treatment plans for optimal patient outcomes. Moreover, as healthcare awareness and infrastructure improve in developing regions, there's a significant opportunity for the market to penetrate these markets, leading to enhanced early detection rates and improved patient care. By leveraging these opportunities through continued research, innovation, and improved healthcare accessibility, the global market for endometrial carcinoma testing by endometrial biopsy stands at the forefront of combating uterine cancer and improving women's health outcomes worldwide.
ENDOMETRIAL CARCINOMA TESTING BY ENDOMETRIAL BIOPSY MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
4.5% |
|
Segments Covered |
By Endometrial Biopsy Devices, End-User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Roche Diagnostics, Abbott Laboratories, BD Biosciences, Hologic, Quest Diagnostics, Siemens Healthineers, Thermo Fisher Scientific, Agilent Technologies |
Global Endometrial Carcinoma Testing By Endometrial Biopsy Market Segmentation: By Endometrial Biopsy Devices
-
Disposable Endometrial Biopsy Catheters
-
Reusable Endometrial Biopsy Devices
The Global Endometrial Carcinoma Testing By Endometrial Biopsy Market is Segmented by Endometrial Biopsy Devices, Disposable Endometrial Biopsy Catheters had the largest market share last year and are poised to maintain their dominance throughout the forecast period. Disposable catheters have emerged as a preferred choice for endometrial biopsy procedures due to several compelling advantages. Firstly, their pre-sterilized nature eliminates the need for time-consuming cleaning and sterilization processes associated with reusable devices, streamlining workflow for healthcare providers and reducing procedure time. Moreover, the single-use nature of disposable catheters significantly reduces the risk of cross-contamination between patients, which is paramount for maintaining patient safety in healthcare settings. Although the upfront cost per device may be higher compared to reusable options, the elimination of expensive sterilization equipment and maintenance expenses can lead to overall cost savings. Additionally, advancements in technology have facilitated the development of cost-effective disposable catheters, making them a viable and attractive option for hospitals and clinics. With a growing emphasis on patient safety and infection control, as well as increased awareness of hospital-acquired infections (HAIs), healthcare facilities are increasingly favoring the use of disposable devices. While reusable devices may offer some cost benefits in the long run for high-volume facilities, the convenience, safety, and overall cost-effectiveness of disposable catheters are likely to drive their continued dominance in the market.
Global Endometrial Carcinoma Testing By Endometrial Biopsy Market Segmentation: By End-User
-
Hospitals
-
Ambulatory Surgical Centers
-
Specialized Gynecological Clinics
The Global Endometrial Carcinoma Testing By Endometrial Biopsy Market is Segmented by End Use, Hospitals had the largest market share last year and are poised to maintain their dominance throughout the forecast period. In the landscape of endometrial carcinoma testing by endometrial biopsy, hospitals are positioned as key players, likely holding the largest market share due to their established infrastructure and comprehensive capabilities. Equipped with specialist gynecologists, pathologists, and advanced imaging technology, hospitals offer a robust environment for performing biopsies, particularly for complex cases that may necessitate additional procedures or specialized care. Additionally, hospitals cater to a broad patient population, including those with pre-existing medical conditions, ensuring a diverse and extensive clientele. However, the emergence of Ambulatory Surgical Centres (ASCs) presents a noteworthy trend, offering outpatient procedures with potential cost savings and faster turnaround times, thus attracting certain patient demographics. Specialized gynecological clinics also serve a niche segment of the market, providing focused care for specific patient needs. While hospitals maintain their dominance, the evolving landscape suggests potential growth opportunities for ASCs and specialized clinics, highlighting the need for ongoing market research to accurately assess market dynamics and distribution.
Global Endometrial Carcinoma Testing By Endometrial Biopsy Market Segmentation: By Region
-
North America
-
Asia-Pacific
-
Europe
-
South America
-
Middle East and Africa
The Global Endometrial Carcinoma Testing By Endometrial Biopsy Market is Segmented by Region, North America had the largest market share last year and are poised to maintain their dominance throughout the forecast period and is poised to maintain its dominance throughout the forecast period. North America holds a strong position in the global endometrial carcinoma testing by endometrial biopsy market, supported by high healthcare expenditure, established diagnostic infrastructure, and heightened awareness of female health issues. However, the landscape is evolving, with the Asia Pacific region, particularly China and India, poised for significant growth due to rising cancer prevalence, increasing disposable income, and improving healthcare infrastructure. While North America may maintain its dominance shortly, the Asia Pacific's rapid growth suggests a potential narrowing of the market share gap in the long run, indicating a shifting dynamic in the global market. Continued monitoring and adaptation to these evolving trends will be crucial for stakeholders to capitalize on emerging opportunities and navigate changing market dynamics effectively.
COVID-19 Impact Analysis on the Global Endometrial Carcinoma Testing By Endometrial Biopsy Market.
The COVID-19 pandemic has significantly impacted the global endometrial carcinoma testing by the endometrial biopsy market, causing disruptions in both supply and demand factors. On the supply side, restrictions on non-essential medical procedures and disruptions in the supply chain led to delays and cancellations of biopsy procedures, reducing the number of tests performed. Additionally, concerns over virus transmission prompted healthcare facilities to prioritize resources for COVID-19 patients, diverting attention and resources away from routine diagnostic procedures. On the demand side, patient reluctance to visit healthcare facilities due to fear of exposure to the virus further dampened market growth. Moreover, economic uncertainties and job losses resulted in financial constraints for individuals, impacting their ability to afford healthcare services, including endometrial carcinoma testing. However, as vaccination efforts progress and healthcare systems adapt to the new normal, the market is expected to gradually recover, with pent-up demand and resumption of postponed procedures driving growth. Furthermore, increased awareness of the importance of cancer screening and early detection, coupled with advancements in telemedicine and home testing options, could reshape the market landscape in the post-pandemic era.
Latest Trends/ Developments:
In the global endometrial carcinoma testing by endometrial biopsy market, several latest trends and developments are shaping the landscape. Firstly, there's a notable shift towards personalized medicine, with increasing emphasis on molecular testing techniques to tailor treatment plans based on individual patient characteristics. This trend is driving the demand for more advanced biopsy tools capable of providing comprehensive molecular insights into endometrial cancer. Additionally, there's growing adoption of liquid biopsy techniques, which analyze circulating tumor DNA in blood samples, offering a non-invasive alternative to traditional tissue biopsies. These liquid biopsies hold promise for improving early detection rates and monitoring treatment response in endometrial carcinoma patients. Moreover, advancements in imaging technology, such as MRI and ultrasound, are enhancing the accuracy of biopsy procedures by providing real-time guidance and visualization during tissue sampling. Furthermore, telemedicine and remote monitoring solutions are gaining traction, enabling greater accessibility to endometrial carcinoma testing services, particularly in underserved or remote regions. Overall, these trends underscore a shift towards more precise, minimally invasive, and accessible diagnostic approaches in the global endometrial carcinoma testing market, ultimately aiming to improve patient outcomes and streamline healthcare delivery.
Key Players:
-
Roche Diagnostics
-
Abbott Laboratories
-
BD Biosciences
-
Hologic
-
Quest Diagnostics
-
Siemens Healthineers
-
Thermo Fisher Scientific
-
Agilent Technologies
Chapter 1. Endometrial Carcinoma Testing By Endometrial Biopsy Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Endometrial Carcinoma Testing By Endometrial Biopsy Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Endometrial Carcinoma Testing By Endometrial Biopsy Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Endometrial Carcinoma Testing By Endometrial Biopsy Market - Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Endometrial Carcinoma Testing By Endometrial Biopsy Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Endometrial Carcinoma Testing By Endometrial Biopsy Market – By Endometrial Biopsy Devices
6.1 Introduction/Key Findings
6.2 Disposable Endometrial Biopsy Catheters
6.3 Reusable Endometrial Biopsy Devices
6.4 Y-O-Y Growth trend Analysis By Endometrial Biopsy Devices
6.5 Absolute $ Opportunity Analysis By Endometrial Biopsy Devices, 2024-2030
Chapter 7. Endometrial Carcinoma Testing By Endometrial Biopsy Market – By End-User
7.1 Introduction/Key Findings
7.2 Hospitals
7.3 Ambulatory Surgical Centers
7.4 Specialized Gynecological Clinics
7.5 Y-O-Y Growth trend Analysis By End-User
7.6 Absolute $ Opportunity Analysis By End-User, 2024-2030
Chapter 8. Endometrial Carcinoma Testing By Endometrial Biopsy Market , By Geography – Market Size, Forecast, Trends & Insights
8.1 North America
8.1.1 By Country
8.1.1.1 U.S.A.
8.1.1.2 Canada
8.1.1.3 Mexico
8.1.2 By Endometrial Biopsy Devices
8.1.3 By End-User
8.1.4 Countries & Segments - Market Attractiveness Analysis
8.2 Europe
8.2.1 By Country
8.2.1.1 U.K
8.2.1.2 Germany
8.2.1.3 France
8.2.1.4 Italy
8.2.1.5 Spain
8.2.1.6 Rest of Europe
8.2.2 By Endometrial Biopsy Devices
8.2.3 By End-User
8.2.4 Countries & Segments - Market Attractiveness Analysis
8.3 Asia Pacific
8.3.1 By Country
8.3.1.1 China
8.3.1.2 Japan
8.3.1.3 South Korea
8.3.1.4 India
8.3.1.5 Australia & New Zealand
8.3.1.6 Rest of Asia-Pacific
8.3.2 By Endometrial Biopsy Devices
8.3.3 By End-User
8.3.4 Countries & Segments - Market Attractiveness Analysis
8.4 South America
8.4.1 By Country
8.4.1.1 Brazil
8.4.1.2 Argentina
8.4.1.3 Colombia
8.4.1.4 Chile
8.4.1.5 Rest of South America
8.4.2 By Endometrial Biopsy Devices
8.4.3 By End-User
8.4.4 Countries & Segments - Market Attractiveness Analysis
8.5 Middle East & Africa
8.5.1 By Country
8.5.1.1 United Arab Emirates (UAE)
8.5.1.2 Saudi Arabia
8.5.1.3 Qatar
8.5.1.4 Israel
8.5.1.5 South Africa
8.5.1.6 Nigeria
8.5.1.7 Kenya
8.5.1.8 Egypt
8.5.1.9 Rest of MEA
8.5.2 By Endometrial Biopsy Devices
8.5.3 By End-User
8.5.4 Countries & Segments - Market Attractiveness Analysis
Chapter 9. Endometrial Carcinoma Testing By Endometrial Biopsy Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
9.1 Roche Diagnostics
9.2 Abbott Laboratories
9.3 BD Biosciences
9.4 Hologic
9.5 Quest Diagnostics
9.6 Siemens Healthineers
9.7 Thermo Fisher Scientific
9.8 Agilent Technologies
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
By 2023, the Global Endometrial Carcinoma Testing By Endometrial Biopsy market is expected to be valued at US$ 33.3 billion.
Through 2030, the global Endometrial Carcinoma Testing By Endometrial Biopsy market is expected to grow at a CAGR of 4.5%.
By 2030, the global Endometrial Carcinoma Testing By Endometrial Biopsy is expected to grow to a value of US$ 45.3 billion.
North America is predicted to lead the market globally for Endometrial Carcinoma Testing By Endometrial Biopsy.
The global Endometrial Carcinoma Testing By Endometrial Biopsy has segments like Endometrial Biopsy Devices, End-use, and Region.