ELISA-based Esoteric Testing Market Size (2024 – 2030)
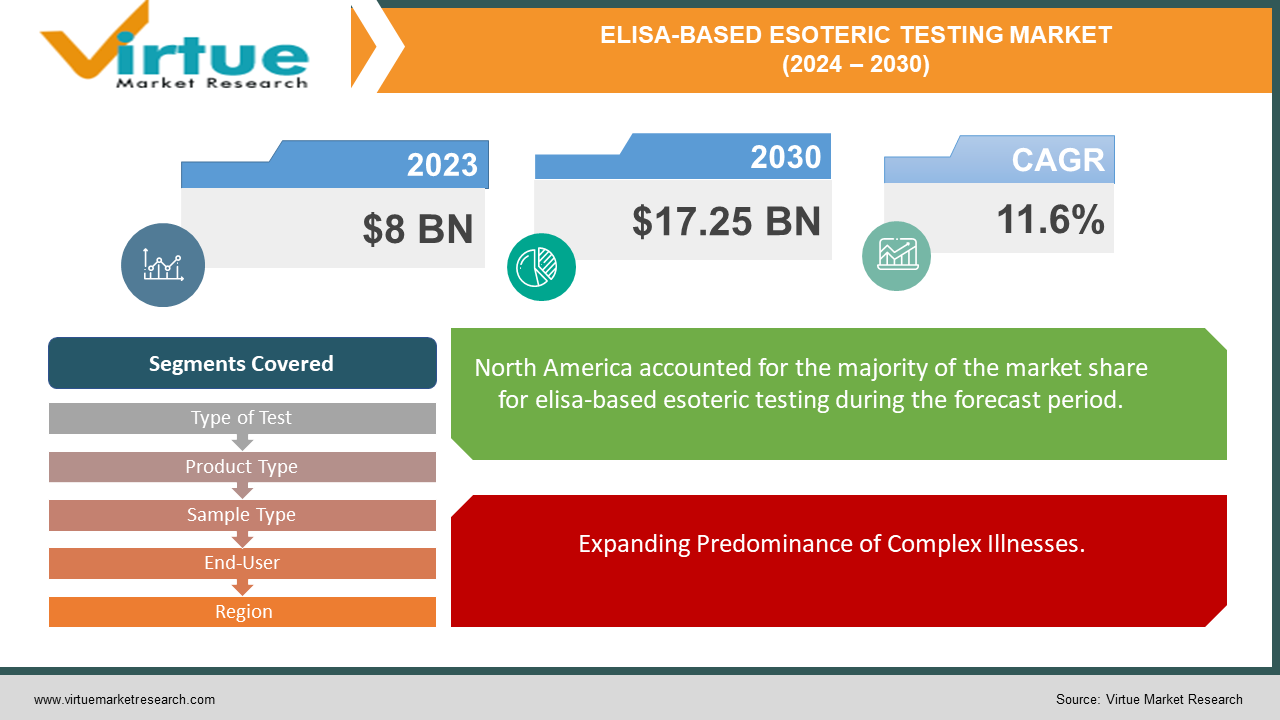
The market for ELISA-based esoteric testing was estimated to be worth 8 USD billion in 2023 and is expected to increase to 17.25 USD billion by 2030, with a projected compound annual growth rate (CAGR) of 11.6% from 2024 to 2030.
The ELISA (Enzyme-Linked Immunosorbent Measure) based obscure testing showcase is seeing strong development, driven by the expanding request for progressed symptomatic devices and the rising predominance of complex illnesses. Exclusive testing alludes to specialized, high-complexity tests that are not routinely performed in clinical research facilities, frequently requiring advanced gear and skill. ELISA, a broadly utilized immunoassay procedure, plays a significant part in this portion due to its affectability, specificity, and flexibility in recognizing and measuring different biomolecules, counting hormones, peptides, antibodies, and antigens. Components such as headways in biotechnology, the developing requirement for early infection discovery, and the development of personalized medication are fueling advertising development. Moreover, the expanded appropriation of ELISA-based tests in investigative teach, symptomatic centers, and healing centers, coupled with the advancement of imaginative ELISA packs for rising maladies and conditions, encourage moves the advertise. Companies that center on advancement, rigid quality control, and growing their test portfolios are well-positioned to capitalize on the developing request in this energetic and competitive advertisement.
Key Insights:
Stem cell therapy accounts for approximately 40% of the total regenerative medicine market, highlighting its significant role in treating a variety of conditions such as cardiovascular diseases, neurological disorders, and musculoskeletal issues.
Over 1,200 regenerative medicine clinical trials are currently active worldwide, demonstrating robust ongoing research efforts and a strong pipeline of potential new therapies.
Despite the market's growth, the high cost of regenerative therapies remains a challenge, with some treatments costing over $100,000.
Global ELISA-based Esoteric Testing Market Drivers:
Expanding Predominance of Complex Illnesses.
The worldwide ELISA-based obscure testing advertise is altogether driven by the rising predominance of complex and incessant illnesses such as cancer, immune system disarranges, and irresistible infections. As the rate of these conditions proceeds to develop, there's an increased request for progressed demonstrative devices that can give precise and early discovery. ELISA-based tests, known for their affectability and specificity, are fundamental in diagnosing and checking these maladies, in this manner fueling advertise development.
Innovative Progressions in ELISA Methods.
Mechanical progressions in ELISA strategies are another key driver of advertising development. Advancements such as multiplex ELISA, which permits the synchronous discovery of numerous analytes, and the advancement of computerized ELISA frameworks upgrade test productivity and precision. These headways not as it were streamline research facility workflows but also decrease turnaround times and the potential for human mistakes, making ELISA-based exclusive testing more alluring to healthcare suppliers and analysts.
Developing Selection of Personalized Medication.
The developing appropriation of personalized pharmaceuticals is additionally moving the request for ELISA-based obscure testing. Personalized pharmaceutical depends on exact symptomatic apparatuses to tailor medicines to personal patients based on their one-of-a-kind hereditary and biochemical profiles. ELISA tests are significant in this approach, as they can degree particular biomarkers and give point-by-point bits of knowledge into a patient's condition. This slant is driving the requirement for more specialized and advanced ELISA tests, subsequently contributing to advertising development.
Global ELISA-based Esoteric Testing Market Restraints and Challenges:
Tall Taken a Toll of Progressed ELISA Tests.
One of the essential restrictions within the worldwide ELISA-based exclusive testing showcase is the tall fetched related to progressed ELISA tests. These tests frequently require advanced gear, specialized reagents, and a gifted workforce, all of which contribute to their by and large cost. The tall costs can be restrictive for numerous healthcare offices, especially in creating districts, constraining the openness and selection of these progressed demonstrative devices. This monetary boundary can moderately advertise development and confine the far-reaching usage of ELISA-based exclusive testing.
Complex Administrative Prerequisites.
The complex and exacting administrative prerequisites for ELISA-based exclusive tests pose a critical challenge to showcase development. These tests must experience thorough approval and endorsement forms to guarantee their precision, unwavering quality, and security. Exploring these administrative scenes can be time-consuming and expensive for producers, deferring the presentation of unused tests to the showcase. Also, shifting controls over diverse nations complicates the worldwide conveyance of ELISA tests, creating obstacles for advertising development.
Restricted Mindfulness and Talented Workforce.
Another noteworthy challenge is the restricted mindfulness and deficiency of a talented workforce capable of ELISA-based obscure testing. In numerous districts, there's a need for satisfactory preparation and instruction regarding the benefits and applications of ELISA tests. Moreover, the complexity of these tests requires profoundly prepared experts to perform and translate them precisely. The shortage of such a talented workforce can ruin the appropriation and viable utilization of ELISA-based testing, especially in resource-limited settings, in this manner limiting showcase development.
Global ELISA-based Esoteric Testing Market Opportunities:
Extension in Rising Markets.
The worldwide ELISA-based obscure testing showcase has critical development potential in developing markets. As the healthcare framework moves forward and mindfulness of progressed symptomatic procedures increments, nations in Asia-Pacific, Latin America, and Africa show considerable openings for showcase extension. Speculation in healthcare offices, coupled with government activities to move forward in healthcare get to, is driving the request for progressed demonstrative instruments like ELISA-based tests in these locales. This extension can be encouraged by associations between neighborhood healthcare suppliers and worldwide demonstrative companies.
Improvement of Point-of-Care ELISA Tests.
The improvement of point-of-care (POC) ELISA tests speaks to a major opportunity for the showcase. These tests, outlined for utilizing exterior conventional research facility settings, give fast come about and are significant for opportune decision-making in clinical settings. Advancements in versatile and user-friendly ELISA gadgets can encourage far-reaching appropriation in farther and resource-limited regions, improving illness determination and administration. The comfort and availability of POC ELISA tests can essentially broaden the market's reach and progress in understanding results.
Integration with Computerized Wellbeing Advances.
Coordination of ELISA-based obscure testing with advanced well-being advances offers a promising opportunity for showcase development. Advanced stages can upgrade the effectiveness and precision of ELISA testing by empowering real-time information examination, further checking, and computerized result translation. This integration can streamline workflows in research facilities and healthcare settings, decrease turnaround times, and make strides in demonstrative exactness. Also, the utilization of manufactured insights and machine learning calculations can improve the prescient capabilities of ELISA tests, giving more profound experiences into infection movement and treatment viability. The meeting of ELISA testing with computerized well-being is balanced to revolutionize the demonstrative scene and drive showcase development.
ELISA-BASED ESOTERIC TESTING MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
11.6% |
|
Segments Covered |
By Type of Test, Product Type, Sample Type, End-User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Thermo Fisher Scientific, Abbott Laboratories, Bio-Rad Laboratories, Roche Diagnostics, Danaher Corporation (Beckman Coulter, Molecular Devices), Siemens Healthineers, PerkinElmer, Merck KGaA (MilliporeSigma), Becton, Dickinson, and Company (BD Biosciences), Ortho Clinical Diagnostics, R&D Systems (A Bio-Techne Brand), Enzo Life Sciences |
ELISA-based Esoteric Testing Market Segmentation: By Type of Test
-
Hormone assays
-
Allergy testing
-
Infectious disease testing (e.g., HIV, hepatitis)
-
Autoimmune disease testing
-
Cancer marker testing
-
Therapeutic drug monitoring
-
Others
Irresistible illness testing is one of the foremost compelling and basic fragments inside the worldwide ELISA-based exclusive testing showcase. This category envelops a wide run of tests for identifying pathogens such as HIV, hepatitis, and different bacterial and viral contaminations. The adequacy of ELISA in irresistible infection testing lies in its tall affectability and specificity, which are significant for precise conclusion and observation. Early and exact discovery of irresistible specialists empowers opportune intercession and treatment, which can altogether diminish the spread of infections and progress quiet results. With the continuous worldwide center on managing infectious infections, especially in the wake of pandemics like COVID-19, the request for solid and fast symptomatic devices has surged. ELISA-based tests, known for their vigor and unwavering quality, are essential in open well-being initiatives and clinical settings, making them a foundation within the battle against irresistible infections. This segment's significance is encourage underscored by persistent mechanical progressions and advancements that upgrade test execution, extend location capabilities, and encourage less demanding and quicker usage in different healthcare situations.
ELISA-based Esoteric Testing Market Segmentation: By Product Type
-
Kits & reagents
-
Analyzers & instruments
-
Software & services
Packs & reagents speak to the foremost successful portion inside the worldwide ELISA-based obscure testing advertise. This item sort is significant since it shapes the establishment of the ELISA testing preparation, giving the vital components for the precise location and evaluation of different analytes in organic tests. Units and reagents are broadly utilized in research facilities and clinical settings due to their flexibility and ease of utilization. They incorporate all the fundamental components such as antigens, antibodies, substrates, and buffers required to perform a total measure. The tall request for units & reagents is driven by their application over a wide range of tests, counting hormone measures, sensitivity testing, irresistible infection testing, immune system illness testing, cancer marker testing, and helpful medicate observing. Additionally, the ceaseless advancement in reagent details and the improvement of ready-to-use packs have essentially improved the unwavering quality, affectability, and specificity of ELISA tests. This has streamlined the workflow in demonstrative research facilities, decreased the time required for test planning, and minimized the potential for blunders. As a result, packs & reagents are crucial in accomplishing steady and reproducible comes about, making them a crucial component within the by and large viability of ELISA-based obscure testing.
ELISA-based Esoteric Testing Market Segmentation: By Sample Type
-
Blood
-
Serum
-
Plasma
-
Urine
-
Saliva
-
Others
Blood is the foremost compelling test sort inside the worldwide ELISA-based obscure testing showcase due to its comprehensive symptomatic capabilities and broad utilization in clinical settings. Blood tests are important for recognizing a wide cluster of biomarkers related to different maladies, counting irresistible illnesses, immune system clutters, cancer markers, and hormonal awkward nature. The tall concentration of analytes in blood permits for exact and delicate location, which is significant for early conclusion and compelling malady checking. Moreover, blood tests can give an all-encompassing see of a patient's well-being status, empowering synchronous testing for numerous conditions employing a single test. This productivity is especially advantageous in overseeing complex illnesses and conditions that require comprehensive screening and observation. The vigor and unwavering quality of blood-based ELISA tests are assisted and upgraded by progressions in blood collection and handling procedures, which minimize test corruption and make strides in test exactness. Given its flexibility and symptomatic accuracy, blood remains the gold standard test sort for ELISA-based exclusive testing, encouraging way better persistent results and progressing the field of demonstrative medication.
ELISA-based Esoteric Testing Market Segmentation: By End-User
-
Hospital diagnostic laboratories
-
Research institutes
-
Pharmaceutical and biotechnology companies
-
Contract research organizations (CROs)
-
Other
Demonstrative research facilities are the foremost viable end-users within the worldwide ELISA-based exclusive testing advertise due to their specialized center on performing complex and high-volume testing with tall exactness and proficiency. These research facilities are prepared with progressed innovations and gifted faculty committed to symptomatic testing, making them the essential settings for conducting a wide run of ELISA tests, counting hormone tests, irresistible malady testing, immune system illness testing, and cancer marker testing. Demonstrative research facilities handle huge volumes of tests, giving quick and solid comes about that are basic for convenient understanding determination and treatment choices. Moreover, symptomatic research facilities regularly serve as central centers for specialized exclusive tests that will not be routinely accessible in smaller clinical settings, this way guaranteeing availability to progressed symptomatic administrations. Their part is pivotal within the healthcare biological system, as they back clinics, clinics, and inquire about establishing by advertising comprehensive testing administrations. The persistent selection of computerization and progressed computer programs in symptomatic research facilities improves the throughput and precision of ELISA tests, guaranteeing consistency and lessening turnaround times. Thus, demonstrative research facilities are crucial within the ELISA-based obscure testing showcase, driving development and guaranteeing the tall measures required for viable malady conclusion and administration.
ELISA-based Esoteric Testing Market Segmentation: Regional Analysis
-
North America
-
Europe
-
Asia-Pacific
-
South America
-
Middle East & Africa
North America holds the biggest share of the worldwide ELISA-based obscure testing showcase, bookkeeping for 40% of the overall advertising. This dominance is driven by the region's progressed healthcare foundation, critical venture in investigation and improvement, and tall appropriation rate of imaginative demonstrative innovations. Europe takes after with a 25% advertise share, profiting from solid healthcare frameworks, broad investigative exercises, and strong administrative systems. The Asia-Pacific locale speaks to 20% of the advertising, appearing to fast development due to expanding healthcare consumption, growing research facility capabilities, and rising mindfulness of progressed diagnostics. South America holds a 9% advertise share, with development driven by progressing healthcare offices and rising requests for progressed demonstrative apparatuses. The Center East and Africa account for the remaining 6%, where advertising extension is backed by progressive healthcare enhancements and developing speculations in the therapeutic framework.
COVID-19 Impact Analysis on the Global ELISA-based Esoteric Testing Market:
The COVID-19 widespread has had a critical effect on the worldwide ELISA-based obscure testing advertise. The pressing requirement for precise and quick demonstrative apparatuses to identify the SARS-CoV-2 infection has driven a surge in requests for ELISA-based tests, as they are exceedingly compelling for recognizing antibodies and antigens related to the infection. This expanded request quickened advancements and headways in ELISA innovation, driving the improvement of more proficient and high-throughput testing arrangements. Moreover, the widespread highlighted the significance of a strong demonstrative framework, inciting considerable speculations in demonstrative research facilities and related offices. Be that as it may, the center on COVID-19 testing briefly occupied assets and consideration from other sorts of exclusive testing, causing delays in scheduled demonstrative administrations for conditions such as cancer and immune system maladies. Despite these challenges, the increased mindfulness and improved symptomatic capabilities cultivated by the widespread are anticipated to have an enduring positive impact on the ELISA-based exclusive testing showcase, driving development and enhancements within the long term.
Latest Trends/ Developments:
The ELISA-based obscure testing showcase is seeing a few key patterns and improvements that are forming its future. One major slant is the integration of computerization and high-throughput advances in ELISA stages, which improves effectiveness and precision, permitting research facilities to handle bigger volumes of tests with minimal manual mediation. Furthermore, there's a developing accentuation on the improvement of point-of-care ELISA tests that give quick and solid results, making progressed diagnostics more open in inaccessible and resource-limited settings. The utilization of advanced well-being technologies, such as AI and machine learning, is additionally on the rise, supporting the elucidation of the complex test comes about and making strides in demonstrative exactness. Another eminent improvement is the extension of ELISA testing applications past conventional regions, such as irresistible infection and hormone tests, to incorporate more current areas like personalized pharmaceutical and biomarker disclosure. Besides, headways in reagent details and the utilization of novel biomaterials are improving the affectability and specificity of ELISA tests. These patterns, combined with expanded speculations in inquiry about and advancement, are driving the showcase forward, guaranteeing proceeded development and made strides persistent results.
Key Players:
-
Thermo Fisher Scientific
-
Abbott Laboratories
-
Bio-Rad Laboratories
-
Roche Diagnostics
-
Danaher Corporation (Beckman Coulter, Molecular Devices)
-
Siemens Healthineers
-
PerkinElmer
-
Merck KGaA (MilliporeSigma)
-
Becton, Dickinson, and Company (BD Biosciences)
-
Ortho Clinical Diagnostics
-
R&D Systems (A Bio-Techne Brand)
-
Enzo Life Sciences
Chapter 1. ELISA-based Esoteric Testing Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. ELISA-based Esoteric Testing Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. ELISA-based Esoteric Testing Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. ELISA-based Esoteric Testing Market Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. ELISA-based Esoteric Testing Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. ELISA-based Esoteric Testing Market – By Type of Test
6.1 Introduction/Key Findings
6.2 Hormone assays
6.3 Allergy testing
6.4 Infectious disease testing (e.g., HIV, hepatitis)
6.5 Autoimmune disease testing
6.6 Cancer marker testing
6.7 Therapeutic drug monitoring
6.8 Others
6.9 Y-O-Y Growth trend Analysis By Type of Test
6.10 Absolute $ Opportunity Analysis By Type of Test, 2024-2030
Chapter 7. ELISA-based Esoteric Testing Market – By Product Type
7.1 Introduction/Key Findings
7.2 Kits & reagents
7.3 Analyzers & instruments
7.4 Software & services
7.5 Y-O-Y Growth trend Analysis By Product Type
7.6 Absolute $ Opportunity Analysis By Product Type, 2024-2030
Chapter 8. ELISA-based Esoteric Testing Market – By Sample Type
8.1 Introduction/Key Findings
8.2 Blood
8.3 Serum
8.4 Plasma
8.5 Urine
8.6 Saliva
8.7 Others
8.8 Y-O-Y Growth trend Analysis By Sample Type
8.9 Absolute $ Opportunity Analysis By Sample Type, 2024-2030
Chapter 9. ELISA-based Esoteric Testing Market – By End-User
9.1 Introduction/Key Findings
9.2 Hospital diagnostic laboratories
9.3 Research institutes
9.4 Pharmaceutical and biotechnology companies
9.5 Contract research organizations (CROs)
9.6 Other
9.7 Y-O-Y Growth trend Analysis By End-User
9.8 Absolute $ Opportunity Analysis By End-User, 2024-2030
Chapter 10. ELISA-based Esoteric Testing Market, By Geography – Market Size, Forecast, Trends & Insights
10.1 North America
10.1.1 By Country
10.1.1.1 U.S.A.
10.1.1.2 Canada
10.1.1.3 Mexico
10.1.2 By Type of Test
10.1.3 By End-User
10.1.4 By Sample Type
10.1.5 Countries & Segments - Market Attractiveness Analysis
10.2 Europe
10.2.1 By Country
10.2.1.1 U.K
10.2.1.2 Germany
10.2.1.3 France
10.2.1.4 Italy
10.2.1.5 Spain
10.2.1.6 Rest of Europe
10.2.2 By Type of Test
10.2.3 By Type
10.2.4 By Sample Type
10.2.5 By End-User
10.2.6 Countries & Segments - Market Attractiveness Analysis
10.3 Asia Pacific
10.3.1 By Country
10.3.1.1 China
10.3.1.2 Japan
10.3.1.3 South Korea
10.3.1.4 India
10.3.1.5 Australia & New Zealand
10.3.1.6 Rest of Asia-Pacific
10.3.2 By Type of Test
10.3.3 By Type
10.3.4 By Sample Type
10.3.5 By End-User
10.3.6 Countries & Segments - Market Attractiveness Analysis
10.4 South America
10.4.1 By Country
10.4.1.1 Brazil
10.4.1.2 Argentina
10.4.1.3 Colombia
10.4.1.4 Chile
10.4.1.5 Rest of South America
10.4.2 By Type of Test
10.4.3 By Type
10.4.4 By Sample Type
10.4.5 By End-User
10.4.6 Countries & Segments - Market Attractiveness Analysis
10.5 Middle East & Africa
10.5.1 By Country
10.5.1.1 United Arab Emirates (UAE)
10.5.1.2 Saudi Arabia
10.5.1.3 Qatar
10.5.1.4 Israel
10.5.1.5 South Africa
10.5.1.6 Nigeria
10.5.1.7 Kenya
10.5.1.8 Egypt
10.5.1.9 Rest of MEA
10.5.2 By Type of Test
10.5.3 By Type
10.5.4 By Sample Type
10.5.5 By End-User
10.5.6 Countries & Segments - Market Attractiveness Analysis
Chapter 11. ELISA-based Esoteric Testing Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
11.1 Thermo Fisher Scientific
11.2 Abbott Laboratories
11.3 Bio-Rad Laboratories
11.4 Roche Diagnostics
11.5 Danaher Corporation (Beckman Coulter, Molecular Devices)
11.6 Siemens Healthineers
11.7 PerkinElmer
11.8 Merck KGaA (MilliporeSigma)
11.9 Becton, Dickinson, and Company (BD Biosciences)
11.10 Ortho Clinical Diagnostics
11.11 R&D Systems (A Bio-Techne Brand)
11.12 Enzo Life Sciences
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The market for ELISA-based esoteric testing was estimated to be worth 8 USD billion in 2023 and is expected to increase to 17.25 USD billion by 2030, with a projected compound annual growth rate (CAGR) of 11.6% from 2024 to 2030.
The essential drivers of the worldwide ELISA-based obscure testing advertise are the expanding predominance of unremitting infections, headways in demonstrative innovations, and developing requests for personalized medication.
Key challenges incorporate competition from elective demonstrative innovations, administrative compliance, test quality issues, tall advancement costs, constrained repayment, and the requirement for exact information translation.
In 2023, North America held the largest share of the global ELISA-based Esoteric Testing market.
Thermo Fisher Scientific, Abbott Laboratories, Bio-Rad Laboratories, Roche Diagnostics, Danaher Corporation, Siemens Healthineers, PerkinElmer, Merck KGaA, Becton, Dickinson and Company (BD Biosciences), Ortho Clinical Diagnostics, R&D Systems, Enzo Life Sciences are the main players.