GLOBAL DYSTONIA DBS DEVICES MARKET SIZE (2023 - 2030)
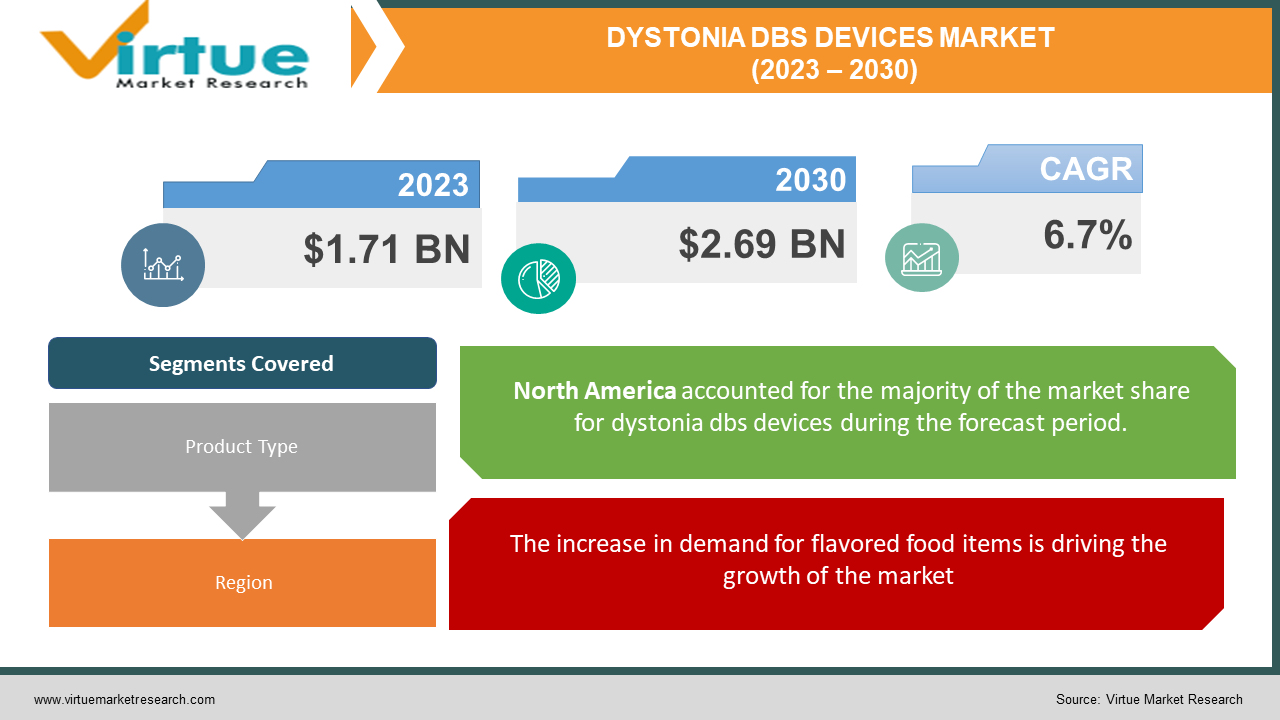
The Global Dystonia DBS Devices Market was valued at USD 1.71 Billion and is projected to reach a market size of USD 2.69 Billion by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 6.7%.
Industry Overview:
Deep brain stimulation (DBS) device, frequently described as an intelligence pacemaker, aids in assuaging signs and symptoms of Parkinson’s disorder (PD). The system is implanted at one of the three U.S. Food and Drug Administration (FDA) accredited intelligence sites to block the electrical alerts from these websites to the brain. The three U.S. FDA-approved centered brain sites are the ventrointermedialis (VIM) nucleus of the thalamus, subthalamic nucleus (STN), and globus pallidus pars interna (GPi). DBS surgical treatment is carried out on sufferers struggling with PD for at least four years and are on medicinal drugs albeit with motor complications. Deep brain stimulation’s blessings over different remedies are projected to supply worthwhile boom potentialities for players in the world market for Parkinson’s disease deep brain stimulation devices. Furthermore, enhancements in electrode plans for deep talent stimulation units for Parkinson’s disorder are projected to raise market growth. Thin-film technology, high-resolution electrodes, and different developments ought to lead to the improvement of multi-contact electrodes that are bendy if needed.
DBS is the most desired option though there are different surgical choices such as Thalamotomy and Pallidotomy, as it does now not contain tissue destruction and is a reversible surgical treatment. The electrodes are accountable for producing electric-powered impulses and assist in controlling the chemical imbalances in the brain. Such impulses are efficaciously managed through a neurostimulator and battery which are implanted in the chest of the patient. DBS is employed to deal with patients struggling with Parkinson’s disease, vital tremors, dystonia, epilepsy, and others.
COVID-19 impact on the Dystonia DBS Devices Market
COVID-19 is an infectious ailment caused by the novel coronavirus. Largely unknown before the outbreak started out in Wuhan (China) in December 2019, COVID-19 modified into an international pandemic in no time. The COVID-19 pandemic has expanded the burden on healthcare structures throughout the globe. Companies scaled up R&D efforts to advance vaccines and drugs in opposition to the virus.
The unexpected outbreak of COVID-19 has offered the world to a standstill. The complete world is struggling with this pandemic with the accelerated burden on hospitals and healthcare professionals. Manufacturers have discovered it challenging to serve their surgeon customers due to the fact some hospitals have confined access to their services or modified access regulations. For instance, in December 2020, in accordance with the document of the National Center of Biotechnology Information, lockdown restriction measures had been related to a subjective worsening of motor and psychiatric signs and symptoms like substantial tiredness, low power, or issues slumbering in Parkinson's Disease and dystonic sufferers handled with DBS, and they may additionally have exacerbated the burden of neurological ailment and improved the continual stress associated to the DBS management.
MARKET DRIVERS:
The increase in demand for flavored food items is driving the growth of the market
The increasing occurrence of PD is predicted to enhance demand for DBS devices. For instance, in accordance with the Parkinson’s disorder Foundation, as of 2022, over 10 million humans are struggling with Parkinson’s disease, worldwide. The findings posted by the company verified that the incidence rate of PD will increase with age and solely 4% of human beings had been identified with PD earlier than the age of 50 years. The increasing incidence of epilepsy is additionally anticipated to improve demand for DBS devices. Nearly 80% of humans with epilepsy stay in low and middle-income nations like India, Kenya, and Bangladesh.
MARKET RESTRAINTS:
Alternative technology present are restraining the growth of the market
The DBS is not only one technique that is prevailing in the market which is helping patients to recover, there are other technologies in the market that are also helping patients to recover from the disease which is restraining the growth of the market during the forecast period. Other recovery methods in the market that are more natural and easier for people to afford are also hindering the growth of the market.
GLOBAL DYSTONIA DBS DEVICES MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2022 - 2030 |
|
Base Year |
2022 |
|
Forecast Period |
2023 - 2030 |
|
CAGR |
6.7% |
|
Segments Covered |
By Product Type and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Boston Scientific Corporation, Medtronic Plc AbbottLaboratories,NeuroPaceInc., Renishaw Plc, Beijing PINS Medical Co. Ltd, LiaNova PLC, Nexstim, Aleva Neurotherapeutics, St. Jude Medical, Sapiens Neuro |
This research report on the global Dystonia DBS Devices Market has been segmented and sub-segmented based on By Product Type, and Region.
Global Dystonia DBS Devices Market - By Product Type:
-
Single-channel Deep Brain Stimulator
-
Dual-channel Deep Brain Stimulator.
Based on Product Type, the dual-channel section dominated the market for deep Genius stimulation gadgets and accounted for an income share of 57.2% in 2022 owing to its greater adoption in surgical procedures. Dual channels are one of the most secure and tremendous units used in surgical procedures. Thus, the excessive occurrence of disabling neurological diseases, a growing variety of surgical strategies for PD, and the developing quantity of hospitals the use dual-channel DBS gadgets are using the segment.
Global Dystonia DBS Devices Market - By Region:
-
North America
-
Europe
-
Asia-Pacific
-
Latin America
-
The Middle East
-
Africa
Geographically, the North American market would take the lead among these. This is due to the increasing lookup and improvement operations in the healthcare sector, which have led to a make bigger improvement in that field. Major companies in the area additionally have a well-established presence in the U.S., which will assist the market is expanding.
Due to the rising demand in countries like Japan, China, and India, the market cost in the Asia Pacific place is predicted to upward push at the quickest rate. due to rising healthcare expenses throughout the region as an end result of rising disposable incomes. The Asia Pacific Dystonia DBS devices market dimension will additionally be boosted through rising public attention to a range of neurological issues like epilepsy, fundamental tremors, and Parkinson's disease. A huge growth-stimulating factor is the increasing availability of scientific amenities such as multispecialty hospitals and forte clinics.
Europe is predicted to have big market growth due to the existence of quite a number of international locations the place persistent neurological troubles are common. The excessive increase in international locations like the UK, Germany, and France will assist the European market in expansion. In distinction to the different areas, it is projected that the deep intelligence stimulation machine market in Latin America would increase very little. However, the enterprise will be pushed in the future by using the rising focus in essential areas like Brazil.
Global Dystonia DBS Devices Market Share by Company
1. Boston Scientific Corporation
2. Medtronic Plc
3. Abbott Laboratories
4. NeuroPace Inc.
5. Renishaw Plc
6. Beijing PINS Medical Co. Ltd
7. LiaNova PLC
8. Nexstim
9. Aleva Neurotherapeutics
10. St. Jude Medical
11. Sapiens Neuro
Recently, Percept, a deep brain stimulation device from Medtronic, was approved by the FDA. BrainSense technology is used by the company to continuously capture, record, and monitor brain signals in individuals with neurological diseases.
A key enterprise approach utilized by using notable purveyors is the acquisition of enterprises that complement a purveyor's present-day goods and services. They are engaged in many activities such as collaboration and promotions which are estimated to drive the market's growth during the forecast period. Key contributors in the food flavor enhancer enterprise use mergers, acquisitions, collaborations, and alliances to extend their operations. The use of new technologies to enhance modern goods, as well as the growth of income and distribution channels, are amongst the systems that key market players are focusing on.
NOTABLE HAPPENINGS IN THE GLOBAL DYSTONIA DBS DEVICES MARKET IN THE RECENT PAST:
-
Approval - In Aug 2022, LivaNova together with MicroPort Scientific received approval for RegaTM pacemakers from the FDA China.
Chapter 1.GLOBAL DYSTONIA DBS DEVICES MARKET – Scope & Methodology
1.1. Market Segmentation
1.2. Assumptions
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2.GLOBAL DYSTONIA DBS DEVICES MARKET – Executive Summary
2.1. Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-19 Impact Analysis
2.3.1. Impact during 2023 - 2030
2.3.2. Impact on Supply – Demand
Chapter 3.GLOBAL DYSTONIA DBS DEVICES MARKET – Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4.GLOBAL DYSTONIA DBS DEVICES MARKET - Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatory Scenario - By Region
4.3 Customer Analysis
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. GLOBAL DYSTONIA DBS DEVICES MARKET - Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6.GLOBAL DYSTONIA DBS DEVICES MARKET – By Product Type:
6.1. Single-channel Deep Brain Stimulator
6.2. Dual-channel Deep Brain Stimulator
Chapter 7.GLOBAL DYSTONIA DBS DEVICES MARKET – By Region
7.1. North America
7.2. Europe
7.3. The Asia Pacific
7.4. Latin America
7.5. The Middle East
7.6. Africa
Chapter 8.GLOBAL DYSTONIA DBS DEVICES MARKET – Company Profiles – (Overview, Product Portfolio, Financials, Developments)
8.1. Boston Scientific Corporation
8.2. Medtronic Plc
8.3. Abbott Laboratories
8.4. NeuroPace Inc
8.5. Renishaw Plc
8.6. Beijing PINS Medical Co. Ltd
8.7. LivaNova PLC
8.8. Nexstim
8.9. Aleva Neurotherapeutics
8.10. St. Jude Medical
8.11. Sapiens Neuro
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The global Dystonia DBS Devices Market size is estimated to grow from USD 1.6 Billion in 2022 to USD 2.69 Billion by 2030. The market is witnessing a healthy CAGR of 6.7% from 2023 to 2030. An increase in the number of patients with epilepsy and Parkinson's disease is the major factor driving the industry's growth.
The Global Dystonia DBS Devices Market drivers are an increase in the number of patients with epilepsy and Parkinson's
The Segments under Global Dystonia DBS Devices Market segments by Product Type are Single-channel Deep Brain Stimulator and Dual-channel Deep Brain Stimulator.
North America is the most dominating region in the Global Dystonia DBS Devices Market
Boston Scientific Corporation, Medtronic Plc, and Abbott Laboratories are the leading players in the Global Dystonia DBS Devices Market.