Dengue Testing Market Size (2025 – 2030)
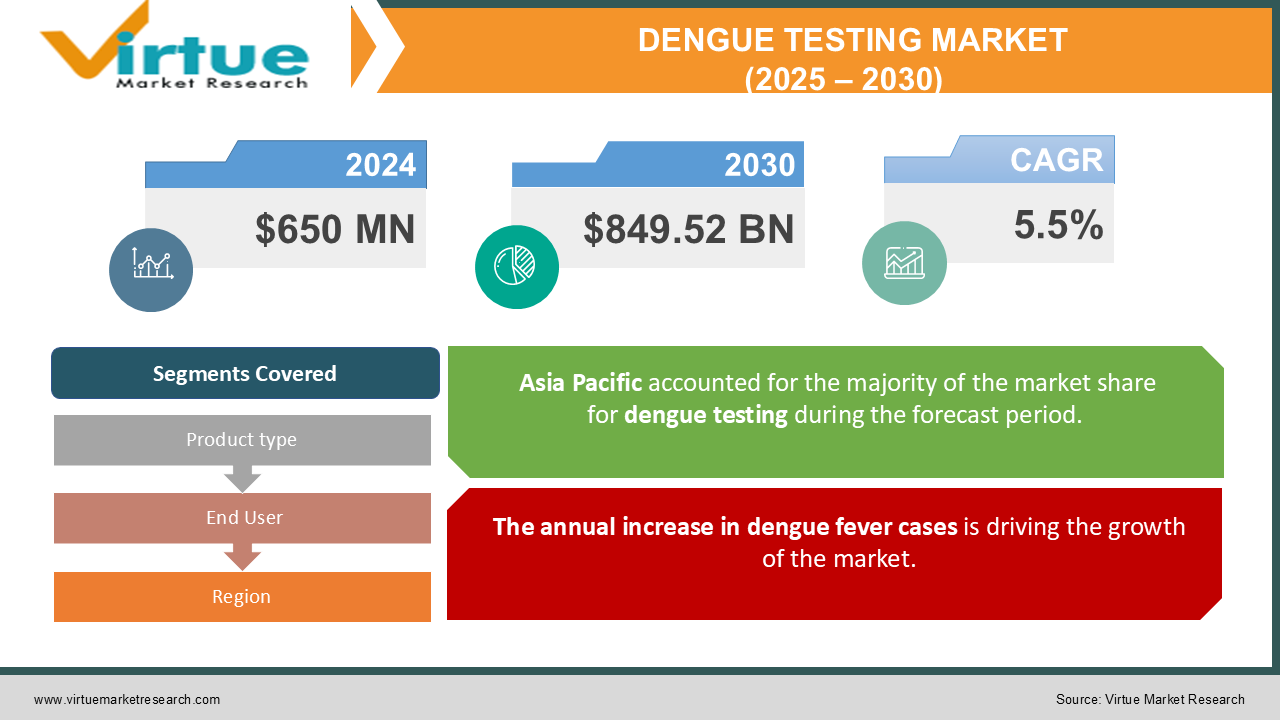
The Dengue Testing Market was valued at USD 650 million in 2024. Over the forecast period of 2025-2030, it is projected to reach USD 849.52 billion by 2030, growing at a CAGR of 5.5%.
Dengue fever is a viral illness transmitted by mosquitoes, impacting individuals. The primary diagnostic methods for detecting the dengue virus include blood tests based on ELISA or PCR techniques. Recently developed rapid point-of-care tests enable the early identification and timely reporting of the disease.
Key Market Insights:
-
The growing prevalence of dengue cases is a key factor fueling the global demand for diagnostic kits.
-
The World Health Organization (WHO) reports a continuous increase in the number of dengue cases.
-
Additionally, technological innovations, enhanced healthcare services, advanced treatments, and government initiatives aimed at raising awareness are all contributing to the expansion opportunities for companies in this field.
-
Manufacturers are prioritizing the development of point-of-care diagnostic kits that are fast, accurate, and user-friendly. As a result, the increasing investment in research and development activities is further propelling the growth of the global dengue testing market.
Dengue Testing Market Drivers:
The annual increase in dengue fever cases is driving the growth of the market.
The growing prevalence of dengue fever, along with increasing awareness initiatives highlighting the disease's impact, is driving the global market for dengue virus diagnostic testing. In recent years, dengue cases have surged significantly. The primary regions affected by dengue include Africa, the Americas, the Eastern Mediterranean, Southeast Asia, and the Western Pacific. Of the reported cases, approximately 500,000 progress to dengue hemorrhagic fever or severe dengue, a more critical form of the disease that results in up to 25,000 deaths annually worldwide.
The demand for affordable test kits with high specificity and sensitivity is rising due to the lack of effective diagnostic tools and the high cost of existing testing solutions. Consequently, companies in the market are focusing on developing cost-effective and reliable dengue diagnostic technologies. Climate change is expected to further increase the incidence of dengue fever, thereby accelerating the growth of the dengue testing market. Despite heightened awareness regarding prevention and treatment, several challenges persist, including insufficient funding and resources, as well as the absence of a clear strategy to tackle the growing challenges posed by dengue outbreaks in various regions. Factors such as rapid urbanization, inadequate sanitation, increased international trade, and population mobility complicate efforts to mitigate the spread of the disease.
The rise in awareness campaigns aimed at educating the public about the dangers of dengue is contributing to the growth of the market. Governments and healthcare organizations worldwide acknowledge the critical role of public awareness in combating dengue. Public health campaigns are designed to educate communities on preventive measures, such as eliminating mosquito breeding sites. These initiatives also emphasize the importance of identifying early symptoms and seeking timely medical care, while fostering community involvement in the effort to fight dengue.
Dengue Testing Market Restraints and Challenges:
The limited availability of testing facilities in remote areas poses a significant barrier to the growth of the market.
The market encounters several challenges, including restricted access to healthcare in remote regions, the high cost of advanced testing kits, and inconsistencies in test accuracy. Additionally, regulatory obstacles and the requirement for skilled professionals complicate the expansion of the market.
Dengue Testing Market Opportunities:
Technological advancements in diagnostic tools present significant opportunities for growth in the market.
Emerging technological advancements have significantly improved the diagnosis of dengue, driving market growth. In particular, point-of-care testing (POCT) offers rapid and more accurate diagnoses in the field of dengue treatment. POCT devices deliver key advantages by providing immediate diagnostic results, which are especially valuable in regions with limited healthcare infrastructure. These tests are quick and convenient, enabling faster decision-making in clinical settings and improving response times during outbreaks. As these technologies continue to evolve, they are becoming more user-friendly and affordable, further promoting their adoption into mainstream healthcare practices worldwide. This trend not only enhances patient care but also plays a crucial role in controlling and reducing disease spread by facilitating timely interventions.
Strategic imperatives are creating valuable opportunities in the market.
To capitalize on regional growth potential, companies must implement localization strategies. Developing diagnostic tests tailored to specific regional dengue strains and aligning with local healthcare systems is essential. Collaborating with local governments, healthcare providers, and partnering with wholesale dengue detection test manufacturers are critical for successful market penetration. Furthermore, the rise of other vector-borne diseases could divert resources, requiring dengue test manufacturers to adopt flexible and adaptable strategies to maintain market relevance.
DENGUE TESTING MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 - 2030 |
|
Base Year |
2024 |
|
Forecast Period |
2025 - 2030 |
|
CAGR |
5.5% |
|
Segments Covered |
By Product type, End User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profile |
Thermo Fisher Scientific Inc., Abbott Laboratories, Roche Molecular Systems Inc. , Abnova Corporation, NovaTec Immundiagnostica GmbH , InBios International, Inc., Euroimmun AG (Sub. PerkinElmer), QuantuMDx Group Ltd, Ceres Nanosciences Inc. , OriGene Technologies |
Dengue Testing Market Segmentation: By product type
-
ELISA-based Tests
-
Dengue IgG/IgM Rapid Test
-
RT-PCR based Tests
-
Others
The ELISA-based segment is anticipated to hold the largest market share throughout the forecast period. The enzyme-linked immunosorbent assay (ELISA), which detects anti-DENV IgM or IgG antibodies in patient serum, remains the most widely used method for diagnosing dengue fever. Early detection and treatment of dengue help reduce morbidity and mortality associated with severe forms of the disease, as well as mitigate the risk of widespread outbreaks. In 2023, the EIA/ELISA category led the immunoassay market, capturing a revenue share of 63.7%. The key advantages of this technology over immunoelectrophoresis and immunodiffusion include reduced test times, quantitative results, and the use of smaller amounts of antisera for analysis.
Meanwhile, RT-PCR testing is expected to experience significant growth. For instance, in April 2022, Beroni Group Limited and Columbia University signed an exclusive licensing agreement, granting Beroni and its affiliates worldwide distribution rights for the patented CII-ArboViroPlex rRT-PCR assay.
Dengue Testing Market Segmentation: By End User
-
Hospitals
-
Diagnostic Centres
-
Others
The ELISA-based segment is projected to maintain a dominant market share throughout the forecast period. The enzyme-linked immunosorbent assay (ELISA), which detects anti-DENV IgM or IgG antibodies in patient serum, is the most widely utilized method for diagnosing dengue fever. Early detection and timely treatment of dengue fever help reduce morbidity and mortality rates associated with severe forms of the disease and minimize the risk of large-scale outbreaks. In 2023, the EIA/ELISA category led the immunoassay market, capturing a revenue share of 63.7%. The technology’s advantages over immunoelectrophoresis and immunodiffusion include faster test times, quantitative results, and the need for smaller amounts of antisera for analysis.
Dengue Testing Market Segmentation- by region
-
North America
-
Europe
-
Asia Pacific
-
South America
-
Middle East & Africa
The Asia Pacific region is expected to lead the global dengue testing market. Dengue fever is most prevalent in Southeast Asia, with the disease continuing to rise. The increasing incidence of infectious diseases, particularly dengue fever, in countries like India, Pakistan, and Sri Lanka, is driving rapid market growth in this region. In the Americas, there were 3.1 million reported cases, with over 25,000 classified as severe. Despite the high number of infections, dengue-related mortality has decreased compared to the previous year.
In Latin America, the market is also experiencing rapid growth due to the rising incidence of dengue fever and government initiatives aimed at improving healthcare infrastructure.
COVID-19 Pandemic: Impact Analysis
The COVID-19 pandemic and subsequent lockdowns led to the closure of airports, ports, and both commercial and domestic transportation in many countries. This had a significant impact on manufacturing activities and operations globally, severely affecting the economies of various nations. The outbreak, along with social distancing measures and restrictions, disrupted business operations and the overall business environment worldwide. The sudden and substantial decline in economic activity resulted in slowdowns across manufacturing, production, agriculture, fisheries, dairy, and other sectors, causing widespread job losses. Supply chain disruptions were further exacerbated by demand-side challenges, including reduced disposable income, dwindling savings, and growing concerns and uncertainties.
Latest Trends/ Developments:
In August 2024, J Mitra & Company launched the Dengue NS1 Antigen self-test kit, marking a first for home use in India. This innovative kit allows individuals to perform the test independently at any location and provides results within 20 minutes through a rapid visual test, simplifying the interpretation of results. The kit is highly sensitive and accurate, capable of detecting all four dengue virus serotypes. Classified as a Point of Care Test (POCT), it is designed for ease of use, requiring no specialized training. The testing process involves placing a drop of blood into the sample well, adding a drop of assay buffer, and receiving results in just 20 minutes.
On September 12, 2024, QIAGEN announced the expansion of its strategic partnership with Bio-Manguinhos/Fiocruz, the primary provider of vaccines and diagnostics for Brazil's Ministry of Health. This expanded collaboration allows Bio-Manguinhos to introduce a sophisticated PCR-based molecular screening platform that detects malaria, HIV, and hepatitis B and C (HBV and HCV). Additionally, this partnership supports Brazil's efforts in monitoring dengue fever by implementing dengue molecular kits, developed with exclusive chemistry created by QIAGEN and Bio-Manguinhos/Fiocruz. QIAGEN will provide essential molecular biology technologies, tailored solutions, and comprehensive training to strengthen Brazil's public health initiatives.
In August 2023, Mylab Discovery Solutions launched two rapid point-of-care dengue tests: the rapid gold test and the high-accuracy dry luminescence assay test. These tests, which provide results in 15-20 minutes, are capable of identifying the stage and progression of dengue infections without the need for laboratory facilities. They are particularly beneficial in resource-limited settings, aiding in dengue screening efforts.
Key Players:
These are top 10 players in the Dengue Testing Market :-
-
Thermo Fisher Scientific Inc.
-
Abbott Laboratories
-
Roche Molecular Systems Inc.
-
Abnova Corporation
-
NovaTec Immundiagnostica GmbH
-
InBios International, Inc.
-
Euroimmun AG (Sub. PerkinElmer)
-
QuantuMDx Group Ltd
-
Ceres Nanosciences Inc.
-
OriGene Technologies
Chapter 1. Dengue Testing Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Dengue Testing Market – Executive Summary
2.1 Market Size & Forecast – (2025 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Dengue Testing Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Dengue Testing Market - Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Dengue Testing Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Dengue Testing Market – By Product Type
6.1 Introduction/Key Findings
6.2 ELISA-based Tests
6.3 Dengue IgG/IgM Rapid Test
6.4 RT-PCR based Tests
6.5 Others
6.6 Y-O-Y Growth trend Analysis By Product Type
6.7 Absolute $ Opportunity Analysis By Product Type, 2025-2030
Chapter 7. Dengue Testing Market – By End User
7.1 Introduction/Key Findings
7.2 Hospitals
7.3 Diagnostic Centres
7.4 Others
7.5 Y-O-Y Growth trend Analysis By End User
7.6 Absolute $ Opportunity Analysis By End User, 2025-2030
Chapter 8. Dengue Testing Market , By Geography – Market Size, Forecast, Trends & Insights
8.1 North America
8.1.1 By Country
8.1.1.1 U.S.A.
8.1.1.2 Canada
8.1.1.3 Mexico
8.1.2 By Product Type
8.1.3 By End User
8.1.4 Countries & Segments - Market Attractiveness Analysis
8.2 Europe
8.2.1 By Country
8.2.1.1 U.K
8.2.1.2 Germany
8.2.1.3 France
8.2.1.4 Italy
8.2.1.5 Spain
8.2.1.6 Rest of Europe
8.2.2 By Product Type
8.2.3 By End User
8.2.4 Countries & Segments - Market Attractiveness Analysis
8.3 Asia Pacific
8.3.1 By Country
8.3.1.1 China
8.3.1.2 Japan
8.3.1.3 South Korea
8.3.1.4 India
8.3.1.5 Australia & New Zealand
8.3.1.6 Rest of Asia-Pacific
8.3.2 By Product Type
8.3.3 By End User
8.3.4 Countries & Segments - Market Attractiveness Analysis
8.4 South America
8.4.1 By Country
8.4.1.1 Brazil
8.4.1.2 Argentina
8.4.1.3 Colombia
8.4.1.4 Chile
8.4.1.5 Rest of South America
8.4.2 By Product Type
8.4.3 By End User
8.4.4 Countries & Segments - Market Attractiveness Analysis
8.5 Middle East & Africa
8.5.1 By Country
8.5.1.1 United Arab Emirates (UAE)
8.5.1.2 Saudi Arabia
8.5.1.3 Qatar
8.5.1.4 Israel
8.5.1.5 South Africa
8.5.1.6 Nigeria
8.5.1.7 Kenya
8.5.1.8 Egypt
8.5.1.9 Rest of MEA
8.5.2 By Product Type
8.5.3 By End User
8.5.4 Countries & Segments - Market Attractiveness Analysis
Chapter 9. Dengue Testing Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
9.1 Thermo Fisher Scientific Inc.
9.2 Abbott Laboratories
9.3 Roche Molecular Systems Inc.
9.4 Abnova Corporation
9.5 NovaTec Immundiagnostica GmbH
9.6 InBios International, Inc.
9.7 Euroimmun AG (Sub. PerkinElmer)
9.8 QuantuMDx Group Ltd
9.9 Ceres Nanosciences Inc.
9.10 OriGene Technologies
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The growing prevalence of dengue cases is a key factor fueling the global demand for diagnostic kits. The World Health Organization (WHO) reports a continuous increase in the number of dengue cases.
The top players operating in the Dengue Testing Market are - Thermo Fisher Scientific Inc., Abbott Laboratories, Roche Molecular Systems Inc., Abnova Corporation and NovaTec Immundiagnostica GmbH.
The COVID-19 pandemic and subsequent lockdowns led to the closure of airports, ports, and both commercial and domestic transportation in many countries.
Emerging technological advancements have significantly improved the diagnosis of dengue, driving market growth. In particular, point-of-care testing (POCT) offers rapid and more accurate diagnoses in the field of dengue treatment.
Latin America is the fastest-growing region in the Dengue Testing Market.