Dendritic Cell Cancer Vaccines Market Size (2025 – 2030)
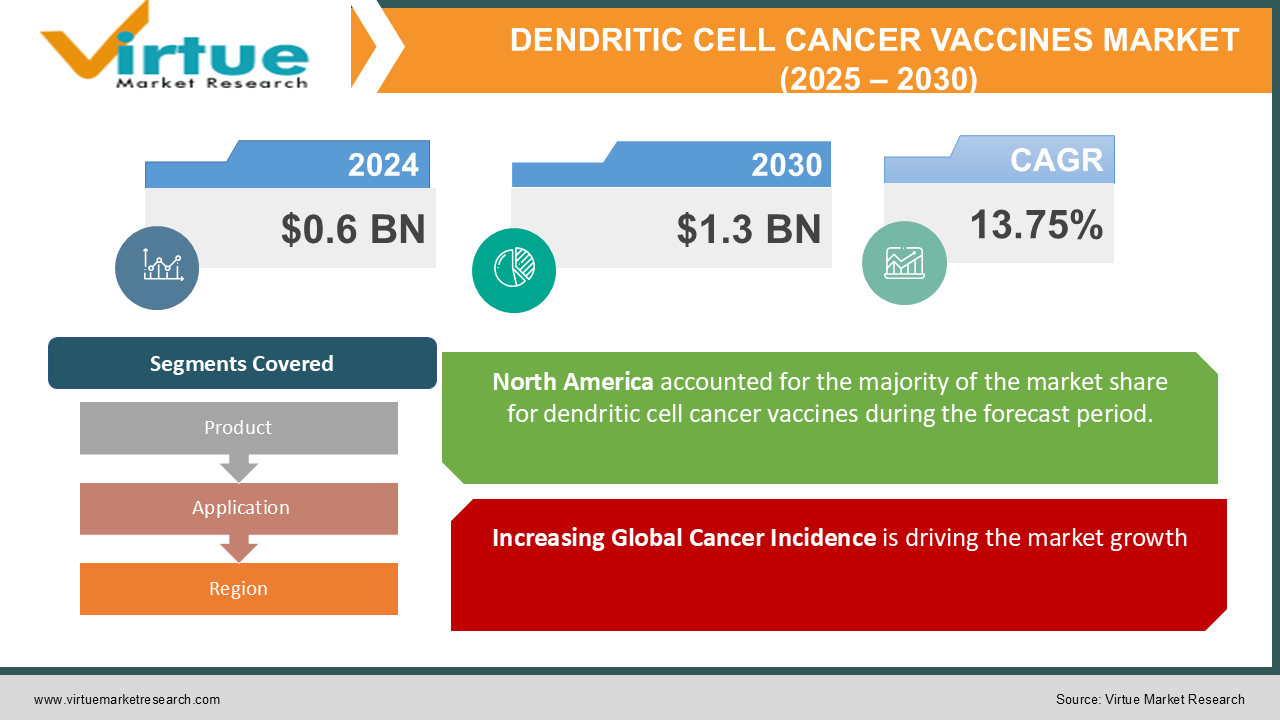
The Global Dendritic Cell Cancer Vaccines Market was valued at USD 0.6 billion in 2024 and is projected to reach USD 1.3 billion by 2030, growing at a CAGR of 13.75% during the forecast period (2025–2030).
Dendritic cell cancer vaccines utilize a patient’s immune system to target and destroy cancer cells, offering an innovative and effective treatment option for various types of cancer.
With a growing global cancer burden and an increasing focus on personalized medicine, dendritic cell vaccines are gaining significant traction. The development of advanced immunotherapy approaches and rising investments in cancer research are expected to drive market growth further.
Key Market Insights
Sipuleucel-T (Provenge) accounted for the largest share of the market in 2024, owing to its regulatory approval for treating prostate cancer.
The prostate cancer segment led the market, representing over 40% of the application share, due to the prevalence of this cancer type among men globally.
Increasing clinical trials and advancements in dendritic cell-based therapies are expected to propel the market significantly. The growing adoption of combination therapies with dendritic cell vaccines is enhancing treatment efficacy, attracting greater interest from oncologists. The rising popularity of personalized medicine is driving demand for dendritic cell vaccines tailored to individual patients' cancer profiles. Cost-related challenges remain significant; however, initiatives to improve access to immunotherapy are mitigating these barriers.
Global Dendritic Cell Cancer Vaccines Market Drivers
Increasing Global Cancer Incidence is driving the market growth
The global cancer burden is rising at an alarming rate, with an estimated 20 million new cases diagnosed annually as of 2024. The growing prevalence of cancers such as prostate cancer, melanoma, and lung cancer highlights the urgent need for innovative and effective treatments. Dendritic cell cancer vaccines, which enhance the body's immune response to target cancer cells, have emerged as a promising therapeutic option.
The advantages of dendritic cell vaccines, including fewer side effects compared to traditional cancer treatments like chemotherapy and radiotherapy, have significantly increased their adoption. Furthermore, the development of vaccines for hard-to-treat cancers, including metastatic cases, is expected to drive demand during the forecast period.
Advances in Immunotherapy and Precision Medicine are driving the market growth
The increasing focus on immunotherapy as a cornerstone of cancer treatment has propelled the demand for dendritic cell cancer vaccines. These vaccines represent a paradigm shift from one-size-fits-all approaches to precision medicine, enabling customized treatments based on patient's genetic and immunological profiles.
Advancements in dendritic cell-based technology, including improved antigen-loading techniques and the use of adjuvants, have enhanced the efficacy of these vaccines. Ongoing research and development (R&D) efforts, supported by significant funding from governments and private entities, are expected to yield innovative solutions and expand the range of treatable cancers.
Regulatory Support and Growing Investments in Cancer Research are driving the market growth
The regulatory landscape for dendritic cell cancer vaccines has become increasingly favorable, with approvals from organizations such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These approvals, coupled with expedited pathways for breakthrough therapies, have encouraged pharmaceutical companies to invest heavily in the development of dendritic cell vaccines.
Global cancer research funding has also seen a significant uptick, enabling the initiation of clinical trials and preclinical studies. This surge in investment is expected to drive innovations and bring more dendritic cell vaccines to market during the forecast period.
Global Dendritic Cell Cancer Vaccines Market Challenges and Restraints
High Costs of Treatment is restricting the market growth
One of the primary challenges facing the dendritic cell cancer vaccine market is the high cost of treatment. The manufacturing process for these vaccines is complex, involving patient-specific cell extraction, modification, and reinfusion. These factors contribute to substantial costs, often making the treatment inaccessible to patients in low- and middle-income countries.
Efforts to reduce costs through advancements in production technologies and government subsidies are underway, but affordability remains a significant barrier. Addressing this challenge will require collaborative efforts among healthcare providers, governments, and pharmaceutical companies to improve affordability and access.
Limited Awareness and Access in Emerging Markets is restricting the market growth
While dendritic cell cancer vaccines are gaining traction in developed regions, awareness, and access remains limited in emerging markets. Factors such as inadequate healthcare infrastructure, lack of trained professionals, and lower cancer diagnosis rates pose challenges for market penetration in regions like Asia-Pacific, Latin America, and Africa. Moreover, cultural and socio-economic barriers further hinder the adoption of advanced cancer treatments in these regions. To overcome these challenges, manufacturers must focus on educational initiatives, partnerships with local healthcare providers, and expanding distribution networks.
Market Opportunities
The global dendritic cell cancer vaccines market offers significant opportunities for growth, driven by the increasing demand for personalized and effective cancer treatments. The potential of these vaccines to target multiple cancer types and their ability to synergize with other treatment modalities present immense scope for innovation. Emerging economies, particularly in Asia-Pacific and Latin America, represent untapped markets due to rising healthcare spending and improving diagnostic capabilities. Manufacturers can capitalize on these opportunities by developing cost-effective solutions and expanding their presence in these regions. Furthermore, collaborations between pharmaceutical companies, research institutions, and healthcare providers can accelerate the development of next-generation dendritic cell vaccines. Advancements in genomic and proteomic technologies are also expected to enhance the customization and efficacy of these vaccines, creating new growth avenues.
DENDRITIC CELL CANCER VACCINES MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2024 - 2030 |
|
Base Year |
2024 |
|
Forecast Period |
2025 - 2030 |
|
CAGR |
13.75% |
|
Segments Covered |
By Product, Application, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Dendreon Pharmaceuticals LLC, Northwest Biotherapeutics, Inc., ImmunoCellular Therapeutics, Ltd., Activartis Biotech GmbH, Medigene AG, Elios Therapeutics, LLC, Argos Therapeutics, Inc., Tella, Inc., CureVac AG, DCPrime BV |
Dendritic Cell Cancer Vaccines Market Segmentation - By Product
-
CreaVax
-
Sipuleucel-T (Provenge)
-
Others
Sipuleucel-T dominated the dendritic cell vaccine market in 2024, primarily due to its regulatory approval and widespread use in the treatment of prostate cancer. As the first FDA-approved dendritic cell vaccine, Sipuleucel-T has established itself as a valuable therapeutic option for patients with advanced prostate cancer. Its unique mechanism of action, which involves stimulating the immune system to target cancer cells, has demonstrated significant clinical benefits in extending overall survival and improving the quality of life for patients. The commercial success of Sipuleucel-T has paved the way for further research and development in the field of dendritic cell vaccines, driving innovation and the emergence of new therapeutic candidates. While other dendritic cell vaccines are in various stages of clinical development, Sipuleucel-T remains a leading product in the market, solidifying its position as a key player in cancer immunotherapy.
Dendritic Cell Cancer Vaccines Market Segmentation - By Application
-
Prostate Cancer
-
Melanoma
-
Others
The prostate cancer segment currently holds the largest share of the dendritic cell vaccine market. This dominance is primarily driven by the high prevalence of prostate cancer globally, particularly among older men. Additionally, the demonstrated efficacy of dendritic cell vaccines in stimulating the immune system to target prostate cancer cells has contributed to their significant role in treatment strategies. These vaccines have shown promising results in clinical trials, leading to their approval by regulatory authorities for specific patient populations. The increasing awareness of prostate cancer and the growing demand for effective treatment options are further propelling the growth of this segment. As research continues to advance and new therapies emerge, the role of dendritic cell vaccines in prostate cancer management is expected to expand, solidifying their position within the market.
Dendritic Cell Cancer Vaccines Market Segmentation - By Region
-
North America
-
Europe
-
Asia-Pacific
-
Latin America
-
Middle East & Africa
North America led the global dendritic cell cancer vaccines market in 2024, contributing to 45% of revenue, due to the region's robust healthcare infrastructure, high R&D investments, and early adoption of advanced therapies. The United States, in particular, is a major contributor, with extensive clinical trials and regulatory approvals driving market growth.
Europe follows closely, supported by increasing cancer incidence and government funding for immunotherapy research. Meanwhile, Asia-Pacific is expected to witness the fastest growth during the forecast period, fueled by improving healthcare infrastructure, rising awareness, and the growing prevalence of cancer in countries such as China, India, and Japan.
COVID-19 Impact Analysis
The COVID-19 pandemic had a mixed impact on the dendritic cell cancer vaccines market. While disruptions in clinical trials and supply chains posed initial challenges, the pandemic also underscored the importance of immunotherapy in healthcare. Cancer patients, being at higher risk of severe COVID-19 complications, highlighted the need for effective treatments that strengthen the immune system. This led to increased interest in immunotherapy, including dendritic cell vaccines, as part of integrated cancer care. Post-pandemic, the market is expected to witness sustained growth, driven by heightened awareness of immune-focused therapies and the resumption of clinical trials and R&D activities.
Latest Trends/Developments
The dendritic cell vaccine market is experiencing significant growth, driven by advancements in technology and increasing recognition of its potential in cancer immunotherapy. Combination therapies, integrating dendritic cell vaccines with immune checkpoint inhibitors and targeted therapies, are emerging as promising approaches to enhance treatment outcomes. Innovations in cell culture and antigen-loading techniques are further improving the efficacy and scalability of these vaccines. As the focus shifts towards rare and hard-to-treat cancers, manufacturers are exploring the potential of dendritic cell vaccines to address unmet medical needs. To capitalize on the growing demand, companies are expanding their market presence into emerging regions like Asia-Pacific and Latin America. Collaborative research and development initiatives between academic institutions and pharmaceutical companies are accelerating vaccine development and fostering innovation in this field. These factors are collectively driving the growth of the dendritic cell vaccine market and positioning it as a key player in the future of cancer immunotherapy.
Key Players
-
Dendreon Pharmaceuticals LLC
-
Northwest Biotherapeutics, Inc.
-
ImmunoCellular Therapeutics, Ltd.
-
Activartis Biotech GmbH
-
Medigene AG
-
Elios Therapeutics, LLC
-
Argos Therapeutics, Inc.
-
Tella, Inc.
-
CureVac AG
-
DCPrime BV
Chapter 1. Dendritic Cell Cancer Vaccines Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Dendritic Cell Cancer Vaccines Market – Executive Summary
2.1 Market Size & Forecast – (2025 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Dendritic Cell Cancer Vaccines Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Dendritic Cell Cancer Vaccines Market - Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Dendritic Cell Cancer Vaccines Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Dendritic Cell Cancer Vaccines Market – By Product
6.1 Introduction/Key Findings
6.2 CreaVax
6.3 Sipuleucel-T (Provenge)
6.4 Others
6.5 Y-O-Y Growth trend Analysis By Product
6.6 Absolute $ Opportunity Analysis By Product, 2025-2030
Chapter 7. Dendritic Cell Cancer Vaccines Market – By Application
7.1 Introduction/Key Findings
7.2 Prostate Cancer
7.3 Melanoma
7.4 Others
7.5 Y-O-Y Growth trend Analysis By Application
7.6 Absolute $ Opportunity Analysis By Application, 2025-2030
Chapter 8. Dendritic Cell Cancer Vaccines Market , By Geography – Market Size, Forecast, Trends & Insights
8.1 North America
8.1.1 By Country
8.1.1.1 U.S.A.
8.1.1.2 Canada
8.1.1.3 Mexico
8.1.2 By Product
8.1.3 By Application
8.1.4 Countries & Segments - Market Attractiveness Analysis
8.2 Europe
8.2.1 By Country
8.2.1.1 U.K
8.2.1.2 Germany
8.2.1.3 France
8.2.1.4 Italy
8.2.1.5 Spain
8.2.1.6 Rest of Europe
8.2.2 By Product
8.2.3 By Application
8.2.4 Countries & Segments - Market Attractiveness Analysis
8.3 Asia Pacific
8.3.1 By Country
8.3.1.1 China
8.3.1.2 Japan
8.3.1.3 South Korea
8.3.1.4 India
8.3.1.5 Australia & New Zealand
8.3.1.6 Rest of Asia-Pacific
8.3.2 By Product
8.3.3 By Application
8.3.4 Countries & Segments - Market Attractiveness Analysis
8.4 South America
8.4.1 By Country
8.4.1.1 Brazil
8.4.1.2 Argentina
8.4.1.3 Colombia
8.4.1.4 Chile
8.4.1.5 Rest of South America
8.4.2 By Product
8.4.3 By Application
8.4.4 Countries & Segments - Market Attractiveness Analysis
8.5 Middle East & Africa
8.5.1 By Country
8.5.1.1 United Arab Emirates (UAE)
8.5.1.2 Saudi Arabia
8.5.1.3 Qatar
8.5.1.4 Israel
8.5.1.5 South Africa
8.5.1.6 Nigeria
8.5.1.7 Kenya
8.5.1.8 Egypt
8.5.1.9 Rest of MEA
8.5.2 By Product
8.5.3 By Application
8.5.4 Countries & Segments - Market Attractiveness Analysis
Chapter 9. Dendritic Cell Cancer Vaccines Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
9.1 Dendreon Pharmaceuticals LLC
9.2 Northwest Biotherapeutics, Inc.
9.3 ImmunoCellular Therapeutics, Ltd.
9.4 Activartis Biotech GmbH
9.5 Medigene AG
9.6 Elios Therapeutics, LLC
9.7 Argos Therapeutics, Inc.
9.8 Tella, Inc.
9.9 CureVac AG
9.10 DCPrime BV
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The market was valued at USD 0.6 billion in 2024 and is projected to reach USD 1.3 billion by 2030, growing at a CAGR of 13.75%.
Key drivers include the increasing global cancer burden, advancements in immunotherapy, and regulatory approvals.
Segments include Product (CreaVax, Sipuleucel-T, Others) and Application (Prostate Cancer, Melanoma, Others).
North America leads the market, accounting for 45% of revenue, driven by advanced healthcare infrastructure and high R&D investments.
Key players include Dendreon Pharmaceuticals LLC, Northwest Biotherapeutics, Inc., and ImmunoCellular Therapeutics, Ltd.



