Cryopreservation Services Market Size (2024 – 2030)
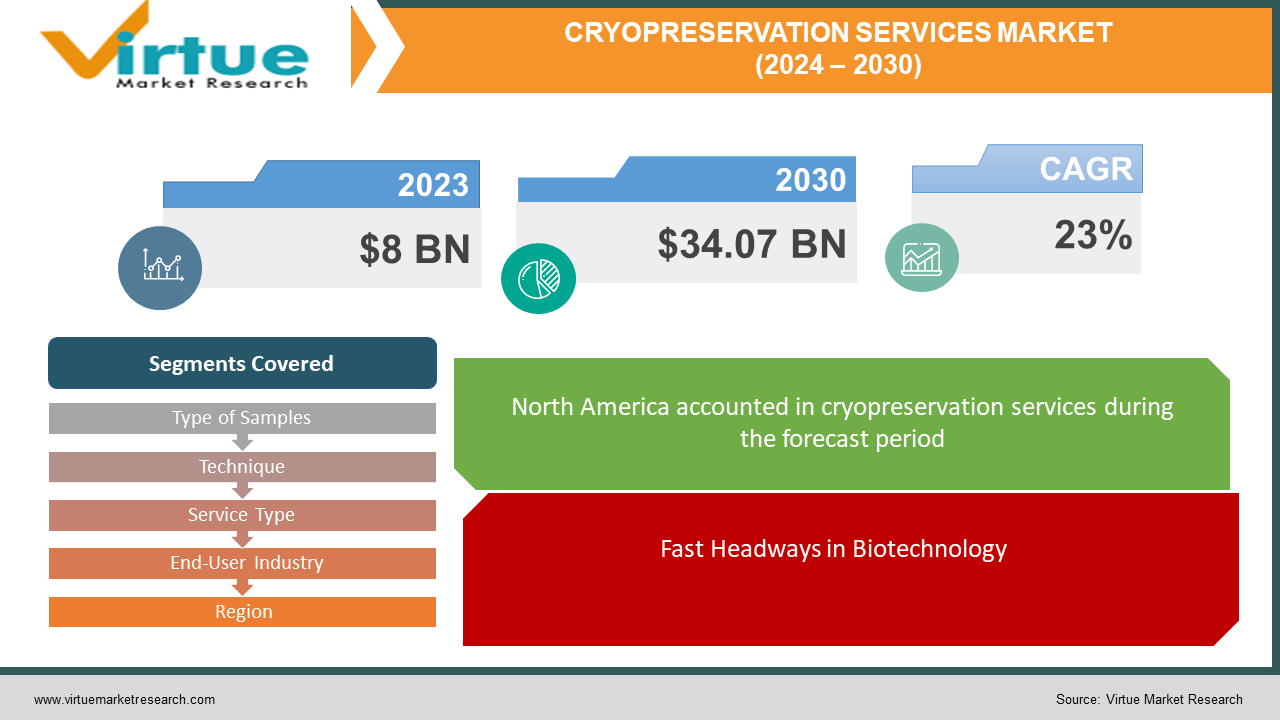
The market for cryopreservation services market at the global level is expanding quickly; it was estimated to be worth 8 USD billion in 2023 and is expected to increase to 34.07 USD billion by 2030, with a projected compound annual growth rate (CAGR) of 23% from 2024 to 2030.
The cryopreservation administration's advertising has seen exceptional development in later a long time, driven by the expanding request for long-term capacity arrangements for natural tests over different businesses. With a center on protecting the reasonability and judgment of cells, tissues, and other natural materials, cryopreservation administrations play a significant part in progressing inquiry about, biotechnology, and healthcare. Components such as rising speculations in stem cell investigation, developing applications in medication disclosure, and the development of personalized medication have impelled the development of this showcase. In addition, innovative progressions in cryopreservation procedures, coupled with exacting controls overseeing test capacity and dealing with it, have assisted fortified advertising development. As we move forward, the cryopreservation administration showcase is balanced for proceeded development, fueled by continuous developments, extending applications, and expanding mindfulness concerning the significance of protecting organic materials for future investigations and restorative endeavors.
Key Market Insights:
The market's extension is outstandingly fueled by the heightening request for personalized medication, coupled with progressions in biotechnology and regenerative medication. Also, the expanding predominance of persistent maladies and the resulting surge in investigative exercises have underscored the significance of productive test capacity and transportation arrangements, encouraging moving to advertise development.Besides, key collaborations among key showcase players, investigative education, and healthcare organizations have encouraged the improvement of imaginative cryopreservation procedures and administrations, upgrading the market's competitiveness.
Global Cryopreservation Services Market Drivers:
Fast Headways in Biotechnology.
The nonstop advancement of biotechnology, especially in areas like regenerative pharmaceutical and stem cell inquiry, could be a major driver for the cryopreservation administration's advertising. Developments in these ranges require solid cryopreservation procedures for the long-term capacity of important natural materials, driving the request for cryopreservation administrations.
Developing Request for Personalized Pharmaceutical.
With the expanding center on personalized medication and exactness treatments, there's an increased require for protecting natural tests such as stem cells and tissues. Cryopreservation administrations empower the capacity of these tests, encouraging investigations and advancement endeavors pointed at fitting medicines to personal patients.
Growing Applications in Sedate Disclosure.
Cryopreserved cell lines play a pivotal part in sedate disclosure and advancement forms, advertising a dependable show for testing the viability and security of unused pharmaceutical compounds. As the pharmaceutical industry proceeds to extend, the request for cryopreservation administrations for putting away cell lines and tissues is anticipated to rise altogether.
Global Cryopreservation Services Market Restraints and Challenges:
Tall Fetched of Cryopreservation Administrations.
One of the essential challenges confronting the cryopreservation administrations showcase is the tall fetched related to cryopreservation methods and capacity offices. This could act as an obstacle for smaller investigations teachers and organizations with constrained budgets, affecting advertising development.
Specialized and Administrative Challenges.
Cryopreservation includes complex specialized forms, and guaranteeing the quality and reasonability of put-away tests can be challenging. Besides, rigid administrative prerequisites overseeing the dealing with and capacity of natural materials include another layer of complexity, posturing obstacles for advertise players.
Moral and Legitimate Contemplations.
Moral concerns encompassing the utilization of cryopreserved organic materials, such as embryos and stem cells, may confine showcase development. Legitimate systems concerning the possession and utilization of put-away samples also pose administrative challenges that have to be tended to by advertising members.
Global Cryopreservation Services Market Opportunities:
Development in Rising Markets.
There's critical undiscovered potential for cryopreservation administrations in rising markets, driven by expanding ventures in healthcare foundation and investigative activities. Advertise players can capitalize on these openings by extending their nearness and advertising custom-made cryopreservation arrangements in these districts.
Innovative Developments.
Ceaseless progressions in cryopreservation methods, counting the improvement of novel cryoprotectants and robotized capacity frameworks, display openings for advertising development. Companies contributing to R&D to make strides in effectiveness, diminish costs, and improve test practicality stand to pick up a competitive edge within advertising.
Key Organizations and Collaborations.
Collaborations between cryopreservation benefit suppliers, inquiries about education, and pharmaceutical companies can open modern openings for advertising development. By leveraging complementary skills and assets, partners can create inventive arrangements and tap into unused markets and applications.
CRYOPRESERVATION SERVICES MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
23% |
|
Segments Covered |
By Type of Samples, Technique, Service Type, End-User Industry, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Thermo Fisher Scientific Inc., Merck KGaA, Charles River Laboratories International, Inc., VWR International, LLC, Custom Biogenic Systems, Inc., Biolife Solutions, Inc., Princeton CryoTech, Inc., StemExpress, LLC, Tissue Solutions Ltd., Biocision, LLC, Sigma-Aldrich Corporation, Brooks Life Sciences |
Cryopreservation Services Market Segmentation: By Type of Samples
-
Cell
-
Tissues
-
Sperms & eggs
-
Embryos
-
Organs
Among the different sections within the cryopreservation administrations advertised categorized by the sort of tests, the conservation of tissues emerges as one of the foremost viable and flexible roads. Tissues include a wide extend of organic materials, including skin, muscle, nerve, and organ tissues, each holding gigantic esteem for inquiry about, transplantation, and helpful applications. Cryopreservation of tissues empowers their long-term capacity while keeping up basic judgment and cellular practicality, making them important assets for restorative investigation and treatment advancement.
Tissue-keeping money encourages progressions in regenerative pharmaceuticals, organ transplantation, and tissue designing, tending to basic healthcare needs such as repairing harmed tissues and organs. In addition, the accessibility of cryopreserved tissues contributes to quickening medicate disclosure forms by giving solid models for testing sedate adequacy and poisonous quality. As a result, the conservation of tissues through cryopreservation administrations not as it were serves as a foundation for therapeutic headways but also offers critical openings for tending to neglected clinical needs and making strides in quiet results.
Cryopreservation Services Market Segmentation: By Technique
-
Slow Freezing: Vitrification
-
Ultra-low Temperature Storage
Inside the advertising division based on method, vitrification stands out as one of the foremost compelling strategies for cryopreservation administrations. Vitrification includes quick cooling of organic tests to amazingly moo temperatures, avoiding the arrangement of ice precious stones, and minimizing cellular harm. This procedure offers a few points of interest over conventional moderate solidifying strategies, counting higher post-thaw cell practicality and progressed conservation of cellular structure and work. Vitrification is especially well-suited for sensitive tests such as embryos, oocytes, and certain sorts of stem cells, where keeping up cellular judgment is foremost. Its productivity in protecting the reasonability of these touchy materials has driven broad appropriation in regenerative advances, stem cell investigation, and tissue designing applications. Furthermore, vitrification empowers the capacity of tests in smaller volumes, diminishing capacity space necessities and related costs. As the request for cryopreservation administrations proceeds to develop, the adequacy and flexibility of vitrification position it as a favored method for protecting profitable organic materials over different businesses, driving market growth and advancement within the field of cryobiology.
Cryopreservation Services Market Segmentation: By Service Type
-
Storage Services
-
Transportation Services
-
Processing Services
Among the divisions based on benefit sort, capacity administrations stand out as the foremost successful and principal component of the cryopreservation administrations. Capacity administrations include the long-term conservation of natural tests at ultra-low temperatures, guaranteeing their reasonability and keenness over expanded periods. These administrations are basic for different businesses, including biotechnology, pharmaceuticals, and inquiries about education, where the conservation of important natural materials is foremost for continuous consideration, future investigative endeavors, and helpful applications. By outsourcing capacity administrations to specialized offices, organizations can advantage of the state-of-the-art foundations, exacting quality control measures, and skill in cryopreservation procedures, in this manner relieving the dangers related to test corruption and misfortune. Besides, the developing request for personalized medication, regenerative treatments, and accurate medications underscores the significance of solid capacity arrangements for natural tests. As such, capacity administrations play a central part in encouraging headways in healthcare, biotechnology, and logical inquiry, driving sustained growth and advancement within the cryopreservation administrations advertise.
Cryopreservation Services Market Segmentation: By End-User Industry
-
Biotechnology and Pharmaceutical Companies
-
Stem Cell Banks
-
Academic and Research Institutes
-
Hospitals and Clinics
Among the portions categorized by end-user industry, biotechnology, and pharmaceutical companies rise as the foremost impactful partners driving the request for cryopreservation administrations. These companies intensely depend on cryopreservation to store different natural tests basic for their inquiry about and advancement endeavors. From protecting cell lines and tissues for sedate disclosure and improvement to keeping money stem cells for restorative applications, cryopreservation administrations play an essential part in supporting the inventive endeavors of biotechnology and pharmaceutical firms. By outsourcing cryopreservation administrations, these companies can center on their center competencies while guaranteeing the long-term practicality and openness of their important natural materials. Additionally, the expanding speculation in biotechnology inquiries and the rising request for personalized medication advance support the centrality of cryopreservation administrations in this segment. As biotech and pharmaceutical companies proceed to initiate headways in healthcare and life sciences, their dependence on viable cryopreservation arrangements is anticipated to drive supported development within the cryopreservation administration's advertisement.
Cryopreservation Services Market Segmentation: Regional Analysis
-
North America
-
Europe
-
Asia-Pacific
-
South America
-
Middle East & Africa
The cryopreservation administration showcase shows an assorted scene of requests over diverse locales, with North America driving the pack as the foremost conspicuous showcase fragment. With a considerable share of 34% in 2023, North America brags a vigorous biotechnology and pharmaceutical division, driving noteworthy requests for cryopreservation administrations. Europe takes after closely behind, speaking to 21% of the advertising, upheld by progressed inquiries about the foundation and a solid accentuation on healthcare advancement. In the interim, the Asia-Pacific locale, comprising 26% of the advertising, illustrates quick development fueled by expanding speculations in healthcare and biotechnology over nations like China, India, and Japan. South America and the Center East and African locales contribute 10% and 9% individually, displaying rising openings in the midst of advancing healthcare scenes and rising mindfulness of cryopreservation advances. Each locale presents an interesting showcase flow formed by components such as administrative systems, mechanical headways, and healthcare consumption, highlighting the significance of region-specific strategies for partners within the cryopreservation administration's showcase.
COVID-19 Impact Analysis on the Global Cryopreservation Services Market:
The COVID-19 widespread has had a multifaceted effect on the worldwide cryopreservation administrations showcase, affecting both request flow and operational hones inside the industry. Whereas the beginning stages of the widespread disturbed supply chains and moderated down non-essential inquiries about exercises, the ensuing accentuation on antibody improvement and restorative inquiries about supported requests for cryopreservation administrations. The critical got to protect natural materials such as viral tests, cell lines, and antibodies for COVID-19 investigation drove expanded utilization of cryopreservation offices.
Furthermore, the widespread underscored the significance of the biobanking and test capacity framework, provoking ventures in improving capacity and guaranteeing the flexibility of cryopreservation operations against future disturbances. Additionally, the appropriation of further observing and automated storage frameworks picked up footing as companies looked to play down onsite faculty and moderate the chance of infection transmission. Looking ahead, the COVID-19 widespread is anticipated to have an enduring effect on the cryopreservation administrations showcase, quickening mechanical advancement and strengthening the centrality of cryopreservation in progressing healthcare readiness and inquiring about capabilities.
Latest Trends/ Developments:
The most recent patterns and advancements within the cryopreservation administrations advertise reflect an energetic scene formed by innovative progressions, advancing inquiries about needs, and moving healthcare ideal models. One unmistakable drift is the expanding selection of mechanized and robotics-assisted cryopreservation frameworks, empowering higher throughput, progressed test consistency, and reduced operational costs. Furthermore, there's a developing center on the improvement of novel cryoprotectants and conservation procedures to improve test reasonability and minimize cellular harm, especially for fragile tests such as embryos and stem cells. Besides, the integration of cryopreservation administrations with developing areas such as quality altering and personalized pharmaceuticals is driving development in test handling and capacity, catering to the advancing needs of biotechnology and pharmaceutical businesses.
Additionally, the COVID-19 widespread has quickened the digitization of cryopreservation forms, driving the selection of inaccessible observing and cloud-based test administration arrangements. These patterns collectively emphasize the industry's commitment to progressing cryopreservation innovations and administrations, guaranteeing the conservation and openness of profitable organic materials for investigation, restorative applications, and healthcare development.
Key Players:
-
Thermo Fisher Scientific Inc.
-
Merck KGaA
-
Charles River Laboratories International, Inc.
-
VWR International, LLC
-
Custom Biogenic Systems, Inc.
-
Biolife Solutions, Inc.
-
Princeton CryoTech, Inc.
-
StemExpress, LLC
-
Tissue Solutions Ltd.
-
Biocision, LLC
-
Sigma-Aldrich Corporation
-
Brooks Life Sciences
Chapter 1. Cryopreservation Services Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Cryopreservation Services Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Cryopreservation Services Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Cryopreservation Services Market Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Cryopreservation Services Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Cryopreservation Services Market – By Type of Samples
6.1 Introduction/Key Findings
6.2 Cell
6.3 Tissues
6.4 Sperms & eggs
6.5 Embryos
6.6 Organs
6.7 Y-O-Y Growth trend Analysis By Type of Samples
6.8 Absolute $ Opportunity Analysis By Type of Samples, 2024-2030
Chapter 7. Cryopreservation Services Market – By Technique
7.1 Introduction/Key Findings
7.2 Slow Freezing: Vitrification
7.3 Ultra-low Temperature Storage
7.4 Y-O-Y Growth trend Analysis By Technique
7.5 Absolute $ Opportunity Analysis By Technique, 2024-2030
Chapter 8. Cryopreservation Services Market – By Service Type
8.1 Introduction/Key Findings
8.2 Storage Services
8.3 Transportation Services
8.4 Processing Services
8.5 Y-O-Y Growth trend Analysis By Service Type
8.6 Absolute $ Opportunity Analysis By Service Type, 2024-2030
Chapter 9. Cryopreservation Services Market – By End-User
9.1 Introduction/Key Findings
9.2 Biotechnology and Pharmaceutical Companies
9.3 Stem Cell Banks
9.4 Academic and Research Institutes
9.5 Hospitals and Clinics
9.6 Y-O-Y Growth trend Analysis By End-User
9.7 Absolute $ Opportunity Analysis By End-User, 2024-2030
Chapter 10. Cryopreservation Services Market, By Geography – Market Size, Forecast, Trends & Insights
10.1 North America
10.1.1 By Country
10.1.1.1 U.S.A.
10.1.1.2 Canada
10.1.1.3 Mexico
10.1.2 By Type of Samples
10.1.3 By Technique
10.1.4 By Service Type
10.1.5 Countries & Segments - Market Attractiveness Analysis
10.2 Europe
10.2.1 By Country
10.2.1.1 U.K
10.2.1.2 Germany
10.2.1.3 France
10.2.1.4 Italy
10.2.1.5 Spain
10.2.1.6 Rest of Europe
10.2.2 By Type of Samples
10.2.3 By Technique
10.2.4 By Service Type
10.2.5 By End-User
10.2.6 Countries & Segments - Market Attractiveness Analysis
10.3 Asia Pacific
10.3.1 By Country
10.3.1.1 China
10.3.1.2 Japan
10.3.1.3 South Korea
10.3.1.4 India
10.3.1.5 Australia & New Zealand
10.3.1.6 Rest of Asia-Pacific
10.3.2 By Type of Samples
10.3.3 By Technique
10.3.4 By Service Type
10.3.5 By End-User
10.3.6 Countries & Segments - Market Attractiveness Analysis
10.4 South America
10.4.1 By Country
10.4.1.1 Brazil
10.4.1.2 Argentina
10.4.1.3 Colombia
10.4.1.4 Chile
10.4.1.5 Rest of South America
10.4.2 By Type of Samples
10.4.3 By Technique
10.4.4 By Service Type
10.4.5 By End-User
10.4.6 Countries & Segments - Market Attractiveness Analysis
10.5 Middle East & Africa
10.5.1 By Country
10.5.1.1 United Arab Emirates (UAE)
10.5.1.2 Saudi Arabia
10.5.1.3 Qatar
10.5.1.4 Israel
10.5.1.5 South Africa
10.5.1.6 Nigeria
10.5.1.7 Kenya
10.5.1.8 Egypt
10.5.1.9 Rest of MEA
10.5.2 By Type of Samples
10.5.3 By Technique
10.5.4 By Service Type
10.5.5 By End-User
10.5.6 Countries & Segments - Market Attractiveness Analysis
Chapter 11. Cryopreservation Services Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
11.1 Thermo Fisher Scientific Inc.
11.2 Merck KGaA
11.3 Charles River Laboratories International, Inc.
11.4 VWR International, LLC
11.5 Custom Biogenic Systems, Inc.
11.6 Biolife Solutions, Inc.
11.7 Princeton CryoTech, Inc.
11.8 StemExpress, LLC
11.9 Tissue Solutions Ltd.
11.10 Biocision, LLC
11.11 Sigma-Aldrich Corporation
11.12 Brooks Life Sciences
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The market for cryopreservation services market at the global level is expanding quickly; it was estimated to be worth 8 USD billion in 2023 and is expected to increase to 34.07 USD billion by 2030, with a projected compound annual growth rate (CAGR) of 23% from 2024 to 2030.
The essential drivers of the Worldwide Cryopreservation Administration Showcase incorporate expanding requests for personalized pharmaceuticals, progressions in biotechnology, and rising applications in sedate revelation and advancement.
The key challenges confronting the Worldwide Cryopreservation Administration Showcase incorporate the tall-taken toll of administrations, specialized and administrative complexities, and moral contemplations.
In 2023, North America held the largest share of the global cryopreservation services Market.
Most players within the cryopreservation administrations advertise incorporate Thermo Fisher Logical Inc., Merck KGaA, Charles Stream Research Facilities Worldwide, Inc., VWR Universal, LLC, Custom Biogenic Frameworks, Inc., Biolife Arrangements, Inc., Princeton CryoTech, Inc., StemExpress, LLC, Tissue Arrangements Ltd., Biocision, LLC, Sigma-Aldrich Enterprise, and Brooks Life Sciences.