Cloud Computing in Clinical Trials Market Size (2024 – 2030)
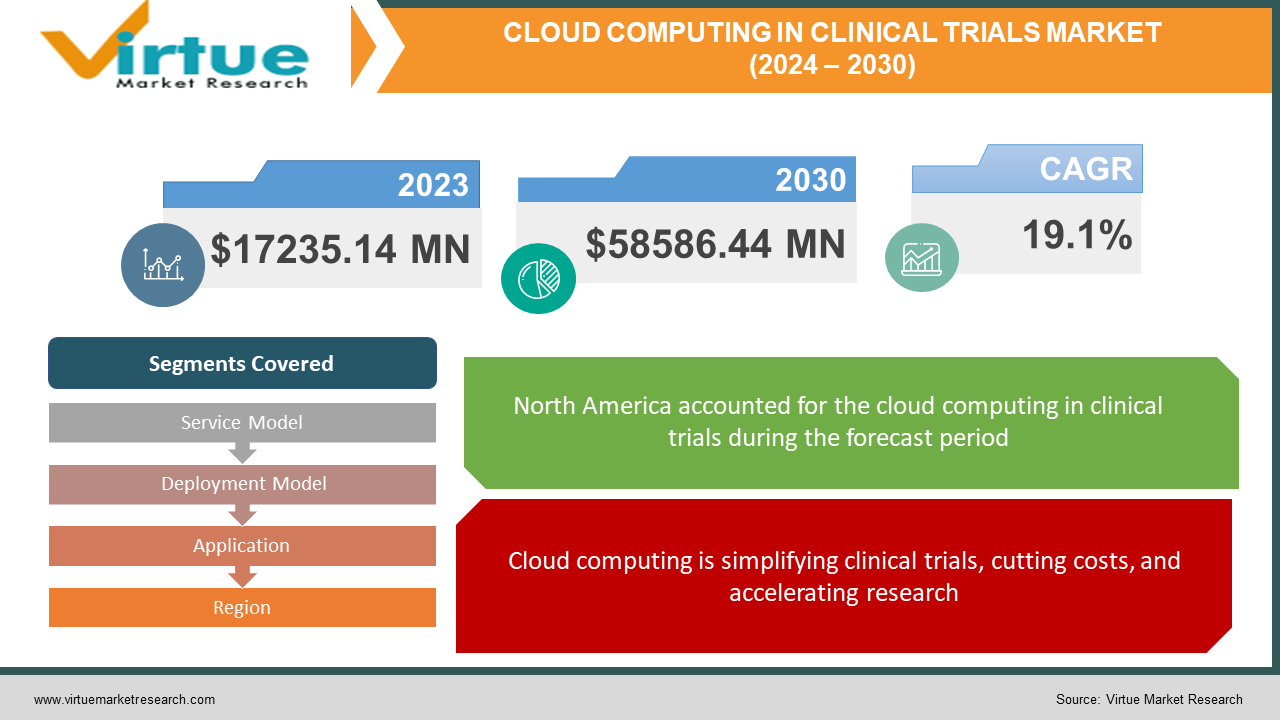
The global Cloud Computing in Clinical Trials market size was exhibited at USD 17235.14 million in 2023 and is projected to hit around USD 58586.44 million by 2030, growing at a CAGR of 19.1% during the forecast period from 2024 to 2030.
Cloud computing is revolutionizing research methodology by providing numerous benefits for clinical trials. Clinical trial management systems (CTMS) that are hosted in the cloud simplify administrative duties and enable smooth communication between researchers from different institutions because of their inherent scalability and collaboration capabilities. Through the elimination of up-front hardware and software costs, cloud providers may provide flexible pay-as-you-go options, which translates to cost savings. In addition, strong security measures protect private patient information, and ongoing surveillance reduces the possibility of security breaches. In the end, cloud computing speeds up clinical trials by facilitating real-time data reporting and analysis, which opens the door for quicker drug development and delivery.
Key Market Insights:
Numerous significant factors are propelling the global clinical trial cloud computing market's growth. Cloud computing provides an appealing alternative to traditional trials, which are costly and slow. It does this by reducing expenses, optimizing workflows, and facilitating remote cooperation. The flexibility and scalability of the cloud are ideal for handling the increasing complexity of drug research, which requires large datasets and substantial processing capacity. Moreover, cloud platforms enable safe collaboration and data sharing across geographically separated researchers, greatly expediting the study process. Sponsors may easily comply with the increasingly stringent regulatory standards for trials thanks to cloud computing's capabilities like secure data storage and audit trails. In addition, a patient-centric strategy is becoming more popular, and cloud-based resources such as mobile apps and patient portals improve patient involvement and trial protocol adherence. Lastly, the strength and effectiveness of cloud platforms for clinical research are continually being increased by developments in cloud technology, such as artificial intelligence and machine learning. But this dynamic economy is not without its difficulties. Due to strict laws and the possibility of data breaches, patient information is still a major worry when it comes to data security and privacy. Collaboration is hampered and data silos are created when there is insufficient connection across various cloud platforms and clinical trial management systems (CTMS). The absence of globally consistent rules about cloud computing in clinical trials may potentially give rise to ambiguity and impede widespread implementation.
Global Cloud Computing in Clinical Trials Market Drivers:
Cloud computing is simplifying clinical trials, cutting costs, and accelerating research.
Traditional clinical trials' exorbitant costs and drawn-out schedules have long been a source of frustration for the medical research community. Cloud computing comes in like a halcyon rider, providing an affordable substitute that expedites procedures and encourages remote cooperation. Cloud computing lowers initial expenses by doing away with the requirement for pricey on-site infrastructure. Furthermore, cloud-based solutions enable effective workflow automation, real-time communication, and data management, greatly simplifying the research process. This results in quicker trial completion timeframes, which in turn speeds up the creation of therapies that can save lives.
Utilising Cloud Computing for Drug Development A Quantum Jump from Scarce Information to Groundbreaking Findings
The field of drug development is going through a significant change. The days of limited computational power and segregated data are long gone. Researchers today deal with an abundance of data, including intricate clinical trial data, genetic sequencing results, and real-world patient data, all of which are essential for the creation of medications that could save lives. Such large datasets demand a robust and adaptable analysis tool. Here's where cloud computing becomes a revolutionary solution. Because of the unmatched scalability provided by cloud platforms, researchers may easily manage ever-increasing data quantities. The constraints of the on-site infrastructure no longer apply to them. Moreover, cloud computing offers the computational power required for intricate analysis. To uncover hidden patterns and reveal the mysteries contained within the data, researchers might make use of cutting-edge algorithms and machine learning technologies. Drug research and development are accelerated by this newfound capacity to extract insightful information from massive information troves, which eventually leads to the faster delivery of novel therapies to patients. Cloud computing opens the door for a future full of ground-breaking medical discoveries by enabling researchers to successfully negotiate the complexity of the data-driven world.
Global Cloud Computing in Clinical Trials Market Restraints and Challenges:
Despite its enormous potential, there are obstacles in the way of the global cloud computing market for clinical trials that must be overcome to ensure further development. Since there is a risk of data breaches when keeping private patient data on the cloud, data security and privacy remain high priorities. Stricter laws such as GDPR and HIPAA also add a level of complexity to guarantee compliance. Another interoperability issue is that different cloud platforms and clinical trial management systems (CTMS) might not be able to communicate with one another well. As a result, data silos are created, making it more difficult for academics worldwide to collaborate. Moreover, the absence of uniform laws in different jurisdictions about cloud computing in clinical trials may confuse and impede implementation worldwide. Another obstacle is vendor lock-in because it can be difficult and costly to switch cloud providers or CTMS, which could impede creativity and flexibility. Another challenge is technical competence since not all research organizations have access to the specialized skills needed to establish and manage cloud-based clinical trial systems. Lastly, even though there may be long-term benefits, the upfront expenses of cloud computing can be high, particularly for smaller organizations. It is impossible to overlook the significant advantages of cloud computing in clinical studies, nevertheless. The industry can get past these obstacles and realize the full promise of this game-changing technology by creating strong security protocols, advocating interoperability standards, and encouraging cooperation across stakeholders.
Global Cloud Computing in Clinical Trials Opportunities:
The market for clinical trials in the cloud is growing globally, and its dynamic nature and increasing usage are creating a wealth of opportunities. The combination of machine learning (ML) and artificial intelligence (AI) on cloud platforms offers promising opportunities for advances in predictive analytics and drug development. With the use of AI, drug development can be sped up by identifying promising drug ideas and improving trial designs through the analysis of enormous datasets. The predictive powers of machine learning in terms of patient reactions and trial outcomes enable more efficient use of resources and reduced risk. Moreover, cloud computing facilitates remote patient monitoring and data collecting, which supports the growth of Decentralised Trials (DCTs). This makes it possible for more patients from different geographical areas to participate who may have previously been unable to do so because of logistical constraints. Furthermore, by collecting data remotely more frequently and putting less strain on patients and study sites, DCTs may increase trial efficiency and hasten trial completion. Cloud-based systems can improve data sharing and collaboration even further between researchers and institutions throughout the globe. Because researchers may more effectively build upon one other's work, there is a greater potential for scientific innovation. Global clinical trials can also be facilitated by providing access to patient populations from different countries. Finally, patient portals and mobile apps are two further ways that cloud computing can be used to enhance the patient experience. Better communication and education may result in greater patient adherence to trial guidelines, which may ultimately improve data quality as data collecting is made easier by user-friendly mobile apps.
CLOUD COMPUTING IN CLINICAL TRIALS MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
19.1% |
|
Segments Covered |
By Service Model, Deployment Model, Application, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Amazon Web Services (AWS), CliniciaRx, Curavit, Empiric Logic, Google Cloud Platform (GCP), IBM Cloud, Medable, Medidata, Microsoft Azure, Oracle Cloud Infrastructure (OCI), POC Pharma, thrive, uMotif, Veeva |
Global Cloud Computing in Clinical Trials Market Segmentation: By Service Model
-
Infrastructure as a Service (IaaS)
-
Platform as a Service (PaaS)
-
Software as a Service (SaaS)
Various important characteristics can be used to categorize the worldwide clinical trial cloud computing market. The service model is the most important of these. SaaS (software as a service) is the largest and fastest expanding segment in this case. Because of its scalability, affordability, and ease of use, software as a service (SaaS) is a desirable alternative for clinical trial management systems (CTMS) and other applications. SaaS is available by subscription. SaaS, in contrast to traditional approaches, allows businesses of all sizes to engage by eliminating the upfront investment in software and equipment. Moreover, SaaS providers make sure users always have access to cutting-edge technology by regularly adding the newest features to their offers. SaaS has become the dominant force in the cloud computing market for clinical trials thanks to its price and flexibility.
Global Cloud Computing in Clinical Trials Market Segmentation: By Deployment Model
-
Public Cloud
-
Private Cloud
-
Hybrid Cloud
Software as a Service (SaaS), which provides clinical trial management tools and applications on a subscription basis, is the industry leader in terms of service models. It is the best because of its scalability, affordability, and ease of use. Public clouds, private clouds, and hybrid clouds are all relevant deployment models. Public clouds are scalable and economical, yet some people have security concerns. While private clouds offer the highest level of protection, they can also be more costly and less scalable. By combining the benefits of both, the hybrid cloud enables businesses to give cost-effectiveness and data sensitivity priority. Even though SaaS is now leading, all market segments have room to develop, and the optimal option will rely on the unique requirements and goals of each organization.
Global Cloud Computing in Clinical Trials Market Segmentation: By Application
-
Clinical Trial Management Systems (CTMS)
-
Electronic Data Capture (EDC)
-
Regulatory Compliance
-
Data Analytics
-
Remote Patient Monitoring (RPM)
The application, or software utilized in the cloud environment, is an additional way to segment the worldwide cloud computing market for clinical trials. The largest category is dominated by Clinical Trial Management Systems (CTMS), which serve as the central system that oversees every facet of a trial. Applications for regulatory compliance and electronic data capture (EDC) come next, guaranteeing data accuracy and legal compliance. Researchers can extract meaningful insights from large datasets with the help of data analytics technologies; the fastest-growing market is remote patient monitoring (RPM). The growth of Decentralised Trials (DCTs), which use RPM to enable distant patient involvement, is the primary cause of this upsurge. We anticipate that the RPM category will continue to increase as DCTs gain popularity.
Global Cloud Computing in Clinical Trials Market Segmentation: By Region
-
North America
-
Asia-Pacific
-
Europe
-
South America
-
Middle East and Africa
North America is a mature market with high adoption rates because of its robust IT infrastructure and support from the government. Currently, the Asia Pacific area is experiencing the fastest growth, nevertheless. China and India are leading the way in this surge, which is being driven by a growing population, rising healthcare spending, and more government support for healthcare innovation. Europe is a seasoned market that places a high priority on data protection and privacy. South America is a developing market with a lot of promise, but it has problems including poor infrastructure and unstable political systems. The Middle East and Africa, on the other hand, have the smallest market, but as their economies rise, so does the interest in cloud computing for clinical trials.
COVID-19 Impact Analysis on the Global Cloud Computing in Clinical Trials Market:
The COVID-19 pandemic catalyzed the growth of the worldwide clinical trial cloud computing business. Travel limits and social distance made remote collaboration and data exchange necessary, which pushed cloud-based systems to the forefront. As a result, the usage of cloud computing in this industry has increased. Moreover, the growth of Decentralised Trials (DCTs) was propelled by the scalability and remote access capabilities of cloud computing. This method made possible by the cloud, increased trial accessibility by enabling patient involvement from remote places, particularly in the event of lockdowns. The pandemic increased concerns about data security, therefore cloud providers prioritized strong security measures like access control and encryption to protect private patient data. In addition, the adoption of real-time data analysis capabilities within cloud platforms was prompted by the necessity of making decisions quickly during the crisis. This made it easier to adjust trials and change directions more quickly in response to new information. In conclusion, it is anticipated that the COVID-19 pandemic will have a long-lasting impact on the clinical trial cloud computing business. Clinical research will probably change dramatically in the future due to the increased reliance on cloud-based solutions for data protection, real-time analytics, and distant collaboration.
Recent Trends and Developments in the Global Cloud Computing in Clinical Trials Market:
The clinical trial cloud computing market is dynamically changing on a global scale. With cloud platforms enabling real-time data analysis and predictive modeling for quicker drug discovery, AI and machine learning are becoming more and more important. With cloud computing enabling secure remote patient monitoring and data gathering, decentralized trials (DCTs) are becoming more popular and more accessible for populations with varying geographic locations. Interoperability between cloud-based systems is becoming increasingly important to guarantee smooth cooperation and remove data silos. Cloud providers are always improving cybersecurity protections with strong encryption, access controls, and compliance with data privacy requirements because they understand how important data privacy is. Lastly, consideration is given to the patient experience. Mobile apps and cloud-based patient portals are being created to enhance patient education and engagement during the trial process, resulting in more precise data collecting. These developments reinforce how the clinical trial cloud computing market is changing and how efficiency, security, and a patient-centered approach are valued most highly.
Key Players:
-
Amazon Web Services (AWS)
-
CliniciaRx
-
Curavit
-
Empiric Logic
-
Google Cloud Platform (GCP)
-
IBM Cloud
-
Medable
-
Medidata
-
Microsoft Azure
-
Oracle Cloud Infrastructure (OCI)
-
POC Pharma
-
thrive
-
uMotif
-
Veeva
Chapter 1. Cloud Computing in Clinical Trials Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Cloud Computing in Clinical Trials Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Cloud Computing in Clinical Trials Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Cloud Computing in Clinical Trials Market Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Cloud Computing in Clinical Trials Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Cloud Computing in Clinical Trials Market – By Service Model
6.1 Introduction/Key Findings
6.2 Infrastructure as a Service (IaaS)
6.3 Platform as a Service (PaaS)
6.4 Software as a Service (SaaS)
6.5 Y-O-Y Growth trend Analysis By Service Model
6.6 Absolute $ Opportunity Analysis By Service Model, 2024-2030
Chapter 7. Cloud Computing in Clinical Trials Market – By Deployment Model
7.1 Introduction/Key Findings
7.2 Public Cloud
7.3 Private Cloud
7.4 Hybrid Cloud
7.5 Y-O-Y Growth trend Analysis By Deployment Model
7.6 Absolute $ Opportunity Analysis By Deployment Model, 2024-2030
Chapter 8. Cloud Computing in Clinical Trials Market – By Application
8.1 Introduction/Key Findings
8.2 Clinical Trial Management Systems (CTMS)
8.3 Electronic Data Capture (EDC)
8.4 Regulatory Compliance
8.5 Data Analytics
8.6 Remote Patient Monitoring (RPM)
8.7 Y-O-Y Growth trend Analysis By Application
8.8 Absolute $ Opportunity Analysis By Application, 2024-2030
Chapter 9. Cloud Computing in Clinical Trials Market , By Geography – Market Size, Forecast, Trends & Insights
9.1 North America
9.1.1 By Country
9.1.1.1 U.S.A.
9.1.1.2 Canada
9.1.1.3 Mexico
9.1.2 By Service Model
9.1.3 By Deployment Model
9.1.4 By Application
9.1.5 Countries & Segments - Market Attractiveness Analysis
9.2 Europe
9.2.1 By Country
9.2.1.1 U.K
9.2.1.2 Germany
9.2.1.3 France
9.2.1.4 Italy
9.2.1.5 Spain
9.2.1.6 Rest of Europe
9.2.2 By Service Model
9.2.3 By Deployment Model
9.2.4 By Application
9.2.5 Countries & Segments - Market Attractiveness Analysis
9.3 Asia Pacific
9.3.1 By Country
9.3.1.1 China
9.3.1.2 Japan
9.3.1.3 South Korea
9.3.1.4 India
9.3.1.5 Australia & New Zealand
9.3.1.6 Rest of Asia-Pacific
9.3.2 By Service Model
9.3.3 By Deployment Model
9.3.4 By Application
9.3.5 Countries & Segments - Market Attractiveness Analysis
9.4 South America
9.4.1 By Country
9.4.1.1 Brazil
9.4.1.2 Argentina
9.4.1.3 Colombia
9.4.1.4 Chile
9.4.1.5 Rest of South America
9.4.2 By Service Model
9.4.3 By Deployment Model
9.4.4 By Application
9.4.5 Countries & Segments - Market Attractiveness Analysis
9.5 Middle East & Africa
9.5.1 By Country
9.5.1.1 United Arab Emirates (UAE)
9.5.1.2 Saudi Arabia
9.5.1.3 Qatar
9.5.1.4 Israel
9.5.1.5 South Africa
9.5.1.6 Nigeria
9.5.1.7 Kenya
9.5.1.8 Egypt
9.5.1.9 Rest of MEA
9.5.2 By Service Model
9.5.3 By Deployment Model
9.5.4 By Application
9.5.5 Countries & Segments - Market Attractiveness Analysis
Chapter 10. Cloud Computing in Clinical Trials Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
10.1 Amazon Web Services (AWS)
10.2 CliniciaRx
10.3 Curavit
10.4 Empiric Logic
10.5 Google Cloud Platform (GCP)
10.6 IBM Cloud
10.7 Medable
10.8 Medidata
10.9 Microsoft Azure
10.10 Oracle Cloud Infrastructure (OCI)
10.11 POC Pharma
10.12 thrive
10.13 uMotif
10.14 Veeva
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The global Cloud Computing in Clinical Trials market size was exhibited at USD 17235.14 million in 2023 and is projected to hit around USD 58586.44 million by 2030, growing at a CAGR of 19.1 % during the forecast period from 2024 to 2030.
The worldwide Global Cloud Computing in Clinical Trials Market growth is estimated to be 19.1 % from 2024 to 2030.
The Global Cloud Computing in Clinical Trials Market is segmented By Service Model (Infrastructure as a Service (IaaS), Platform as a Service (PaaS), Software as a Service (SaaS)), By Deployment Model (Public Cloud, Private Cloud, Hybrid Cloud), By Application (Clinical Trial Management Systems (CTMS), Electronic Data Capture (EDC), Regulatory Compliance, Data Analytics, Remote Patient Monitoring (RPM)) and By Region.
AI and machine learning (AI/ML) integration for data analysis and predictive modeling is anticipated to soar in the clinical trial cloud computing industry. Processes for drug development and discovery will be accelerated by this. Furthermore, cloud-based technologies will accelerate the implementation of decentralized clinical trials (DCTs) by facilitating patient involvement from remote places and improving trial accessibility.
Because the COVID-19 pandemic required remote collaboration and quicker research, cloud computing deployment in clinical trials was greatly boosted. Cloud-based solutions become indispensable for researchers to share data and carry out studies remotely due to social distancing measures and travel limitations. It is anticipated that this increase in demand will have a long-term effect, and that cloud computing will become even more important in the next clinical studies.