Chapter 1. Global Clinical Trial Management System Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
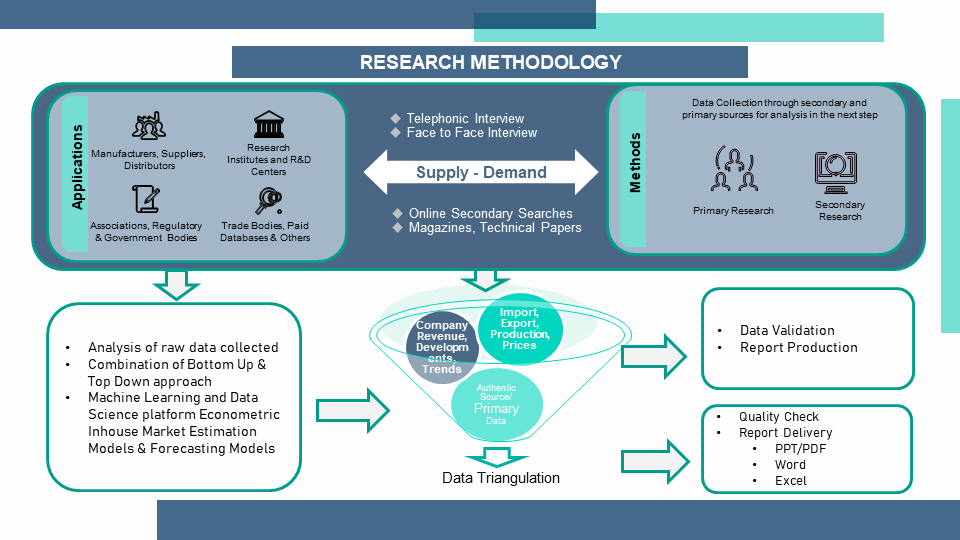
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2.Global Clinical Trial Management System Market– Executive Summary
2.1 Market Size & Forecast – (2022 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Global Clinical Trial Management System Market– Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Global Clinical Trial Management System MarketEntry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
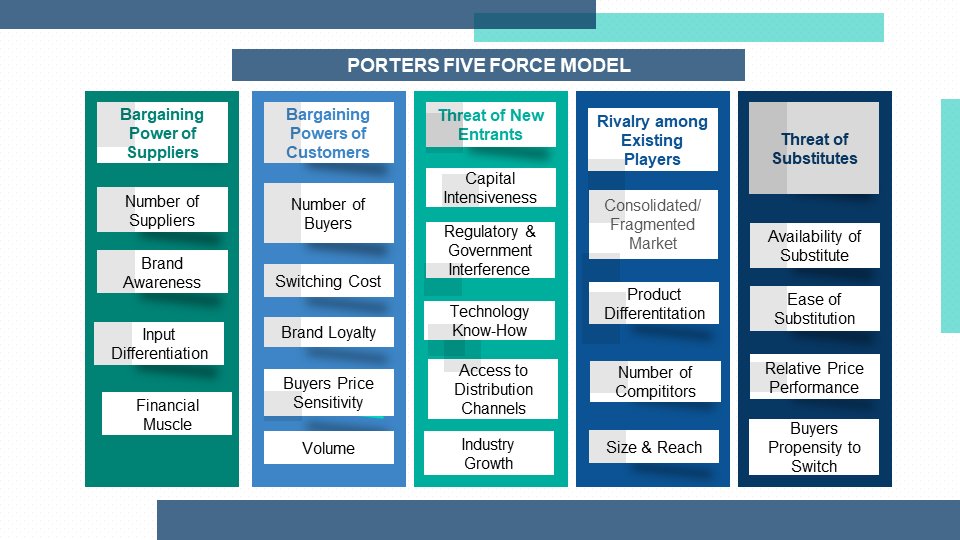
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Global Clinical Trial Management System Market– Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Global Clinical Trial Management System Market– By Type
6.1 Introduction/Key Findings
6.2 Enterprise-Based
6.3 Site-Based
6.4 Y-O-Y Growth trend Analysis By Type
6.5 Absolute $ Opportunity Analysis By Type, 2023-2030
Chapter 7. Global Clinical Trial Management System Market– By Delivery Mode
7.1 Introduction/Key Findings
7.2 Web-based CTMS
7.3 On-premise
7.4 Cloud Based
7.5 Y-O-Y Growth trend Analysis By Delivery Mode
7.6 Absolute $ Opportunity Analysis By Delivery Mode, 2023-2030
Chapter 8. Global Clinical Trial Management System Market– By Component
8.1 Introduction/Key Findings
8.2 Software
8.3 Services
8.4 Y-O-Y Growth trend Analysis By Component
8.5 Absolute $ Opportunity Analysis By Component, 2023-2030
Chapter 9. Global Clinical Trial Management System Market– By End-User
9.1 Introduction/Key Findings
9.2 Large Pharma-biotech Companies
9.3 CROs
9.4 Medical Device Manufacturers
9.5 Small & Mid-sized Pharma-biotech Companies
9.6 Others
9.7 Y-O-Y Growth trend Analysis By End-User
9.8 Absolute $ Opportunity Analysis By End-User, 2023-2030
Chapter 10. Global Clinical Trial Management System Market– By Functionality
10.1 Introduction/Key Findings
10.2 Study Design
10.3 Study Planning and Management
10.4 Patient Data Management
10.5 Site Management
10.6 Trial and Project Tracking
10.7 Regulatory Reporting
10.8 Resource Management
10.9 Y-O-Y Growth trend Analysis By Functionality
10.10 Absolute $ Opportunity Analysis By Functionality, 2023-2030
Chapter 11. Global Clinical Trial Management System Market– By Clinical Trial Phase
11.1 Introduction/Key Findings
11.2 Phase I
11.3 Phase II
11.4 phase III
11.5 Phase IV
11.6 Y-O-Y Growth trend Analysis By Clinical Trial Phase
11.7 Absolute $ Opportunity Analysis By Clinical Trial Phase, 2023-2030
Chapter 12. Global Clinical Trial Management System Market, By Geography – Market Size, Forecast, Trends & Insights
12.1 North America
12.1.1 By Country
12.1.1.1 U.S.A.
12.1.1.2 Canada
12.1.1.3 Mexico
12.1.2 By Type
12.1.2.1 By Delivery Mode
12.1.3 By Component
12.1.4 By Functionality
12.1.5 By Clinical Trial Phase
12.1.6 Countries & Segments - Market Attractiveness Analysis
12.2 Europe
12.2.1 By Country
12.2.1.1 U.K
12.2.1.2 Germany
12.2.1.3 France
12.2.1.4 Italy
12.2.1.5 Spain
12.2.1.6 Rest of Europe
12.2.2 By Type
12.2.3 By Delivery Mode
12.2.4 By Component
12.2.5 By End-User
12.2.6 By Functionality
12.2.7 By Clinical Trial Phase
12.2.8 Countries & Segments - Market Attractiveness Analysis
12.3 Asia Pacific
12.3.1 By Country
12.3.1.1 China
12.3.1.2 Japan
12.3.1.3 South Korea
12.3.1.4 India
12.3.1.5 Australia & New Zealand
12.3.1.6 Rest of Asia-Pacific
12.3.2 By Type
12.3.3 By Delivery Mode
12.3.4 By Component
12.3.5 By End-User
12.3.6 By Functionality
12.3.7 By Clinical Trial Phase
12.3.8 Countries & Segments - Market Attractiveness Analysis
12.4 South America
12.4.1 By Country
12.4.1.1 Brazil
12.4.1.2 Argentina
12.4.1.3 Colombia
12.4.1.4 Chile
12.4.1.5 Rest of South America
12.4.2 By Type
12.4.3 By Delivery Mode
12.4.4 By Component
12.4.5 By End-User
12.4.6 By Functionality
12.4.7 By Clinical Trial Phase
12.4.8 Countries & Segments - Market Attractiveness Analysis
12.5 Middle East & Africa
12.5.1 By Country
12.5.1.1 United Arab Emirates (UAE)
12.5.1.2 Saudi Arabia
12.5.1.3 Qatar
12.5.1.4 Israel
12.5.1.5 South Africa
12.5.1.6 Nigeria
12.5.1.7 Kenya
12.5.1.8 Egypt
12.5.1.9 Rest of MEA
12.5.2 By Type
12.5.3 By Delivery Mode
12.5.4 By Component
12.5.5 By End-User
12.5.6 By Functionality
12.5.7 By Clinical Trial Phase
12.5.8 Countries & Segments - Market Attractiveness Analysis
Chapter 13. Global Clinical Trial Management System Market– Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
13.1. IQVIA Inc.
13.2. Advarra
13.3. Oracle
13.4. DATATRAK International, Inc
13.5. Clario
13.6. Company 6
13.7. Company 7
13.8. Company 8
13.9. Company 9
13.10. Company 10
Download Sample
Choose License Type
2500
4250
5250
6900



