Clinical Laboratory Services For Predictive & Presymptomatic Testing Market Size (2024-2030)
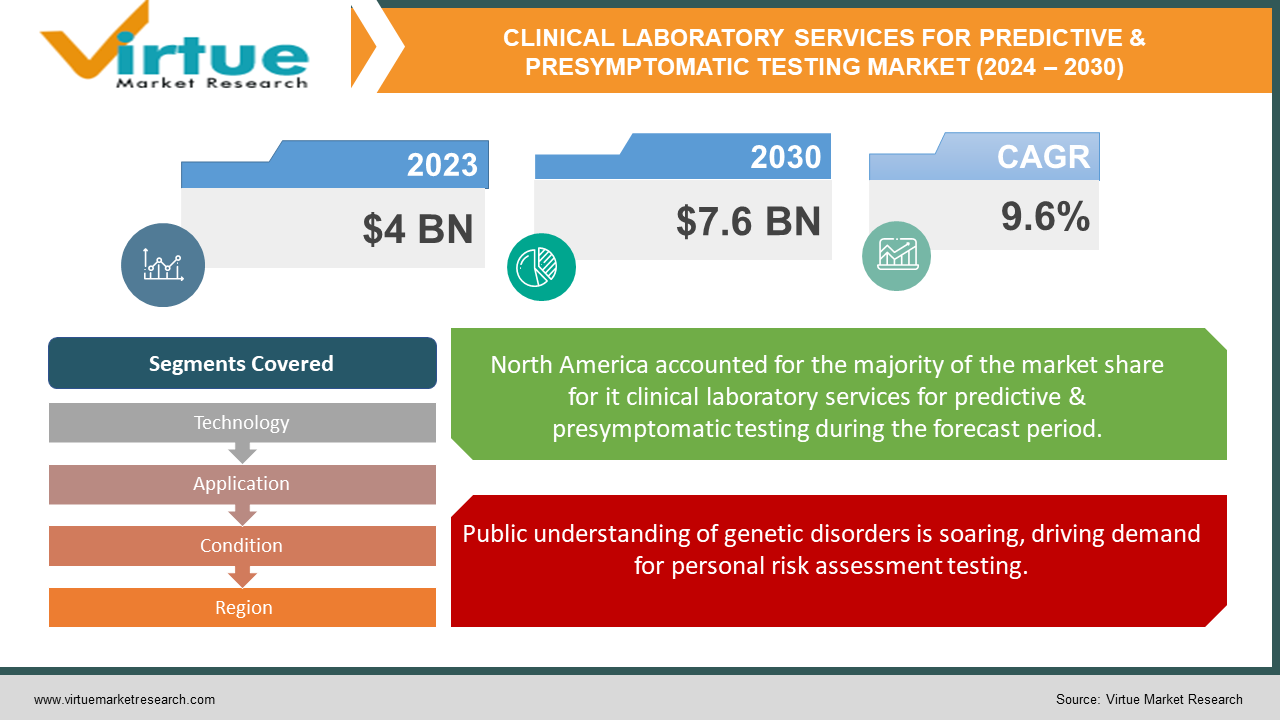
The Clinical Laboratory Services For Predictive & Presymptomatic Testing Market was valued at USD 4 billion in 2023 and is projected to reach a market size of USD 7.6 billion by the end of 2030. Over the cast period of 2024 – 2030, the figure for requests is projected to grow at a CAGR of 9.6%.
The clinical laboratory services market for predictive and presymptomatic testing is experiencing a surge. This growth is fueled by a perfect storm of factors. Firstly, a growing number of people are becoming aware of genetic disorders, leading them to seek testing to assess their risk. Secondly, advancements in genetic testing technologies are making it possible to test for a wider range of conditions more accurately and affordably.
Key Market Insights:
This market is brimming with exciting possibilities. Technological advancements are making genetic testing more powerful than ever. This opens the door for a significant portion of the population to benefit from these tests.
However, alongside this growth, there are important considerations. Ethical concerns surrounding genetic testing and the potential for discrimination based on results require careful attention and clear guidelines. Therefore, establishing clear regulations for interpreting tests and responsibly using the results is essential for the ethical and responsible implementation of this rapidly growing field.
The Clinical Laboratory Services For Predictive & Presymptomatic Testing Market Drivers:
Public understanding of genetic disorders is soaring, driving demand for personal risk assessment testing.
Public knowledge surrounding genetic disorders is experiencing a rapid ascent. This heightened awareness is largely fueled by media coverage, educational campaigns, and the growing availability of direct-to-consumer genetic testing options. As a result, a significantly larger number of individuals are actively seeking testing to understand their personal risk factors for various diseases, propelling the demand for clinical laboratory services in this area.
New and advanced genetic testing methods offer greater accuracy and affordability, expanding accessibility.
The field of genetic testing is undergoing a transformative revolution, characterized by the development and implementation of new and advanced methods. These advancements allow for a wider range of conditions to be tested for with greater accuracy and affordability compared to traditional methods. This not only improves the effectiveness of the tests but also opens the door for a much larger portion of the population to benefit from predictive and presymptomatic testing, fostering significant growth within the market.
The growing focus on early disease detection and prevention fuels demand for predictive testing for better patient outcomes.
The healthcare landscape is witnessing a significant shift towards preventive measures. People are increasingly recognizing the advantages of early disease detection and prevention, driven by factors like rising healthcare costs and a growing emphasis on overall well-being. Predictive testing aligns perfectly with this trend by allowing for earlier intervention in the disease process. By identifying individuals at an increased risk for specific conditions, healthcare professionals can implement preventative strategies and tailor treatment plans, potentially leading to better patient outcomes and reduced healthcare costs in the long run.
The rise of personalized medicine increases demand for genetic insights to tailor treatment plans.
The rise of personalized medicine is another key driver propelling the market forward. Predictive and presymptomatic testing can provide valuable insights into an individual's unique genetic makeup. This information empowers healthcare professionals to tailor treatment plans and preventative strategies based on a patient's specific needs and risk factors identified through genetic testing. This personalized approach to healthcare holds immense promise for improving patient outcomes and overall health management.
The Clinical Laboratory Services For Predictive & Presymptomatic Testing Market Restraints and Challenges:
The burgeoning Clinical Laboratory Services for Predictive & Presymptomatic Testing Market faces challenges alongside its exciting growth. Ethical considerations surrounding genetic testing are paramount. Concerns exist regarding potential discrimination based on test results, particularly in areas like employment or insurance. To mitigate these concerns, clear guidelines and regulations are needed to ensure the responsible use of genetic information. Equitable access to testing is another hurdle. While affordability is improving, cost can still be a barrier for some populations. Ensuring underserved communities have access to testing is crucial to maximize the benefits of this market.
Standardization and regulations are also essential. As the field rapidly evolves, clear regulations and standardized practices are needed. Addressing these challenges will be crucial for ensuring the responsible and equitable implementation of this promising field.
The Clinical Laboratory Services For Predictive & Presymptomatic Testing Market Opportunities:
The Clinical Laboratory Services for Predictive & Presymptomatic Testing Market brims with exciting opportunities. Emerging markets like Asia-Pacific and Latin America present significant growth potential as healthcare infrastructure improves and awareness about genetic testing rises. Clinical laboratory services can tap into this growing demand by expanding their presence in these regions. Collaboration is also key. Fostering partnerships between genetic testing labs, research institutions, and pharmaceutical companies can accelerate innovation and market penetration. This collaboration can lead to the development of more advanced and targeted tests, while pharmaceutical companies can leverage genetic data to develop personalized treatments.
The growing field of pharmacogenomics, which studies how genes influence medication response, presents another opportunity. These methods are less invasive and more convenient compared to traditional tissue biopsies, potentially leading to wider adoption.
CLINICAL LABORATORY SERVICES FOR PREDICTIVE & PRESYMPTOMATIC TESTING MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
9.6% |
|
Segments Covered |
By Technology, Application, Condition, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Abbott Laboratories, Quest Diagnostics Inc., Myriad Genetics, 23andMe, Positive Biosciences, Color Genomics, Gene by Gene, Mapmygenome, Ambry Genetics Corporation, Access Company |
Clinical Laboratory Services for Predictive & Presymptomatic Testing Market Segmentation: By Technology
-
Karyotyping
-
Polymerase Chain Reaction
-
Next-Generation Sequencing
Currently, Karyotyping (traditional chromosomal analysis) is the most dominant technology segment in the Clinical Laboratory Services for Predictive & Presymptomatic Testing Market. However, Next-Generation Sequencing (NGS) is experiencing the fastest growth due to its ability to analyse a broader range of genetic information. This makes NGS a powerful tool for identifying individuals at risk for a wider variety of conditions.
Clinical Laboratory Services for Predictive & Presymptomatic Testing Market Segmentation: By Application
-
Pre-symptomatic testing
-
Carrier screening
-
Pharmacogenomics
The most dominant segment by application in the Clinical Laboratory Services for Predictive & Presymptomatic Testing Market is likely pre-symptomatic testing. This segment focuses on identifying individuals at risk of developing diseases before they show any symptoms, allowing for early intervention and potentially better outcomes. The fastest-growing segment is expected to be pharmacogenomics. This rapidly developing field tailors medication based on an individual's genetic makeup, aiming to improve treatment efficacy and reduce side effects.
Clinical Laboratory Services for Predictive & Presymptomatic Testing Market Segmentation: By Condition
-
Cancer
-
Cardiovascular disease
-
Neurological disorders
-
Infectious diseases
-
Carrier screening
While specific market research may point to variations, dominant and fastest-growing segments likely differ. The most dominant segment by condition is likely Cancer (breast, ovarian, colon, etc.) due to its prevalence and established testing options. The fastest-growing segment is expected to be Pharmacogenomics, driven by the increasing focus on personalized medicine and the potential for tailored treatment plans based on an individual's genetic makeup.
Clinical Laboratory Services for Predictive & Presymptomatic Testing Market Segmentation: Regional Analysis
-
North America
-
Europe
-
Asia-Pacific
-
South America
-
Middle East and Africa
North America currently holds the dominant position in the market due to several factors. Advanced healthcare infrastructure, high disposable income, and a strong focus on preventive healthcare contribute to its leadership. Additionally, a well-established regulatory framework and significant investment in research and development further drive the market in North America.
Asia-Pacific represents a significant growth opportunity due to its rapidly developing healthcare infrastructure and increasing awareness about genetic testing. The growing middle class with rising disposable income is another factor propelling the market forward. However, challenges like uneven healthcare infrastructure development and limited access to advanced testing facilities in some parts of Asia-Pacific need to be addressed to unlock its full potential.
COVID-19 Impact Analysis on the Clinical Laboratory Services For Predictive & Presymptomatic Testing Market:
The COVID-19 pandemic's impact on the Clinical Laboratory Services for Predictive & Presymptomatic Testing Market proved to be a complex one. In the initial stages, the market witnessed a significant downturn. Lockdowns and a necessary shift in healthcare priorities towards tackling the pandemic led to a decline in demand for non-essential medical services, including some predictive and presymptomatic testing procedures. This decline was further compounded by the reallocation of crucial laboratory resources towards COVID-19 testing, limiting capacity for other types of analyses.
However, as the pandemic progressed, some long-term effects emerged that might positively impact the market in the coming years. The unprecedented public health crisis has undoubtedly heightened public awareness of health issues and the importance of preventive measures.
Overall, while the COVID-19 pandemic caused a temporary disruption to the Clinical Laboratory Services for Predictive & Presymptomatic Testing Market, the long-term outlook appears positive. The potential for increased demand driven by a growing awareness of preventive healthcare and potential applications in managing post-COVID conditions suggests a promising future for this market.
Latest Trends/ Developments:
The Clinical Laboratory Services for Predictive & Presymptomatic Testing Market is experiencing a wave of exciting new developments. Non-invasive liquid biopsy methods, often using simple blood tests, are gaining traction. This offers a more convenient and less intrusive alternative to traditional tissue biopsies, potentially increasing the appeal of predictive testing. Additionally, the field is witnessing the growing integration of Artificial Intelligence (AI). AI algorithms can analyze vast amounts of genetic data, leading to more accurate and personalized risk assessments. This information can also be used to identify new potential targets for treatments. Furthermore, the rise of Direct-to-Consumer (DTC) genetic testing kits empowers individuals to access genetic testing directly. Finally, there's a growing focus on improving access and representation in clinical trials for minority populations. This ensures that tests are accurate and effective for a wider range of people, promoting equitable access to the benefits of predictive and presymptomatic testing.
Key Players:
-
Abbott Laboratories
-
Quest Diagnostics Inc.
-
Myriad Genetics
-
23andMe
-
Positive Biosciences
-
Color Genomics
-
Gene by Gene
-
Mapmygenome
-
Ambry Genetics Corporation
-
Access Company
Chapter 1. Clinical Laboratory Services For Predictive & Presymptomatic Testing Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Clinical Laboratory Services For Predictive & Presymptomatic Testing Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Clinical Laboratory Services For Predictive & Presymptomatic Testing Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Clinical Laboratory Services For Predictive & Presymptomatic Testing Market Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Clinical Laboratory Services For Predictive & Presymptomatic Testing Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Clinical Laboratory Services For Predictive & Presymptomatic Testing Market – By Technology
6.1 Introduction/Key Findings
6.2 Karyotyping
6.3 Polymerase Chain Reaction
6.4 Next-Generation Sequencing
6.5 Y-O-Y Growth trend Analysis By Technology
6.6 Absolute $ Opportunity Analysis By Technology, 2024-2030
Chapter 7. Clinical Laboratory Services For Predictive & Presymptomatic Testing Market – By Application
7.1 Introduction/Key Findings
7.2 Pre-symptomatic testing
7.3 Carrier screening
7.4 Pharmacogenomics
7.5 Y-O-Y Growth trend Analysis By Application
7.6 Absolute $ Opportunity Analysis By Application, 2024-2030
Chapter 8. Clinical Laboratory Services For Predictive & Presymptomatic Testing Market – By Condition
8.1 Introduction/Key Findings
8.2 Cancer
8.3 Cardiovascular disease
8.4 Neurological disorders
8.5 Infectious diseases
8.6 Carrier screening
8.7 Y-O-Y Growth trend Analysis By Condition
8.8 Absolute $ Opportunity Analysis By Condition, 2024-2030
Chapter 9. Clinical Laboratory Services For Predictive & Presymptomatic Testing Market , By Geography – Market Size, Forecast, Trends & Insights
9.1 North America
9.1.1 By Country
9.1.1.1 U.S.A.
9.1.1.2 Canada
9.1.1.3 Mexico
9.1.2 By Technology
9.1.3 By Application
9.1.4 By By Condition
9.1.5 Countries & Segments - Market Attractiveness Analysis
9.2 Europe
9.2.1 By Country
9.2.1.1 U.K
9.2.1.2 Germany
9.2.1.3 France
9.2.1.4 Italy
9.2.1.5 Spain
9.2.1.6 Rest of Europe
9.2.2 By Technology
9.2.3 By Application
9.2.4 By Condition
9.2.5 Countries & Segments - Market Attractiveness Analysis
9.3 Asia Pacific
9.3.1 By Country
9.3.1.1 China
9.3.1.2 Japan
9.3.1.3 South Korea
9.3.1.4 India
9.3.1.5 Australia & New Zealand
9.3.1.6 Rest of Asia-Pacific
9.3.2 By Technology
9.3.3 By Application
9.3.4 By Condition
9.3.5 Countries & Segments - Market Attractiveness Analysis
9.4 South America
9.4.1 By Country
9.4.1.1 Brazil
9.4.1.2 Argentina
9.4.1.3 Colombia
9.4.1.4 Chile
9.4.1.5 Rest of South America
9.4.2 By Technology
9.4.3 By Application
9.4.4 By Condition
9.4.5 Countries & Segments - Market Attractiveness Analysis
9.5 Middle East & Africa
9.5.1 By Country
9.5.1.1 United Arab Emirates (UAE)
9.5.1.2 Saudi Arabia
9.5.1.3 Qatar
9.5.1.4 Israel
9.5.1.5 South Africa
9.5.1.6 Nigeria
9.5.1.7 Kenya
9.5.1.8 Egypt
9.5.1.9 Rest of MEA
9.5.2 By Technology
9.5.3 By Application
9.5.4 By Condition
9.5.5 Countries & Segments - Market Attractiveness Analysis
Chapter 10. Clinical Laboratory Services For Predictive & Presymptomatic Testing Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
10.1 Abbott Laboratories
10.2 Quest Diagnostics Inc.
10.3 Myriad Genetics
10.4 23andMe
10.5 Positive Biosciences
10.6 Color Genomics
10.7 Gene by Gene
10.8 Mapmygenome
10.9 Ambry Genetics Corporation
10.10 Access Company
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The Clinical Laboratory Services For Predictive & Presymptomatic Testing Market was valued at USD 4 billion in 2023 and is projected to reach a market size of USD 7.6 billion by the end of 2030. Over the cast period of 2024 – 2030, the figure for requests is projected to grow at a CAGR of 9.6%.
Soaring Public Awareness, Technological Revolution, Preventive Healthcare Focus, Growing Demand for Personalized Medicine.
Karyotyping, Polymerase Chain Reaction, Next-Generation Sequencing.
Currently, the most dominant region for the Clinical Laboratory Services for Predictive & Presymptomatic Testing Market is North America, due to factors like advanced healthcare infrastructure, high disposable income, and a strong focus on preventive healthcare.
Abbott Laboratories, Quest Diagnostics Inc., Myriad Genetics, 23andMe, Positive Biosciences, Color Genomics, Gene by Gene, Mapmygenome, Ambry Genetics Corporation, Access Company.