Cell and Gene Therapy for Musculoskeletal Diseases Market Size (2023-2030)
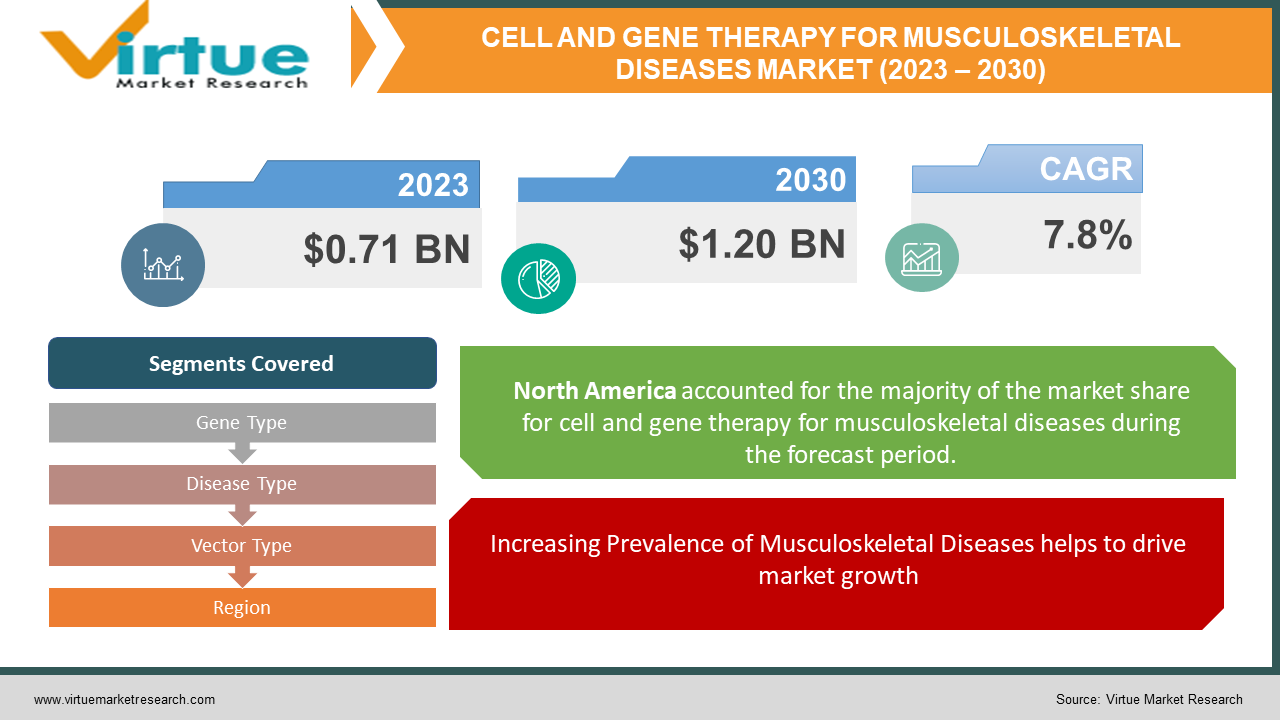
The Global Cell and Gene Therapy for Musculoskeletal Diseases Market was valued at USD 0.71 Billion and is projected to reach a market size of USD 1.20 Billion by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 7.8%.
Industry Overview:
The field of cell and gene therapy for musculoskeletal diseases is rapidly evolving, driven by the potential of these therapies to provide long-lasting, disease-modifying effects. Musculoskeletal diseases, such as osteoarthritis & rheumatoid arthritis, are highly prevalent and a major cause of disability worldwide. Cell and gene therapies offer the potential to address the underlying causes of these diseases, rather than simply managing symptoms. Recent advancements in gene editing and gene delivery technologies have enabled the development of more sophisticated cell and gene therapies for musculoskeletal diseases. These therapies include chimeric antigen receptor (CAR) T-cell therapies, gene therapies targeting specific proteins involved in disease pathology, & mesenchymal stem cell therapies that have immunomodulatory and regenerative properties. The cell and gene therapy for musculoskeletal diseases market is highly competitive, with a growing number of companies investing in research and development in this area. However, the market also faces significant regulatory and reimbursement challenges, which could impact the pace of commercialization of these therapies.
COVID-19 impact on the Cell and Gene Therapy for Musculoskeletal Diseases Market:
The COVID-19 pandemic has had a significant impact on the Cell and Gene Therapy for Musculoskeletal Diseases Market. The pandemic has caused disruptions in the supply chain of raw materials and a delay in clinical trials, affecting the production and development of cell & gene therapies. Additionally, the diversion of resources and funding towards the pandemic has reduced investment in the research and development of cell and gene therapies for musculoskeletal diseases. However, the pandemic has also brought attention to the importance of innovative therapies, leading to increased investment in the field. The shift towards telemedicine has also facilitated patient access to therapies and clinical trials. Moreover, the pandemic has highlighted the need for efficient & safe treatments for musculoskeletal diseases, increasing the demand for cell and gene therapies in the market. Overall, the impact of the pandemic on the Cell and Gene Therapy for Musculoskeletal Diseases Market has been a mix of challenges and opportunities.
Market Drivers:
- Increasing Prevalence of Musculoskeletal Diseases helps to drive market growth: -
- One of the major drivers for the cell and gene therapy for musculoskeletal diseases market is the increasing prevalence of musculoskeletal disorders such as osteoarthritis, rheumatoid arthritis, & spinal cord injuries. According to the World Health Organization (WHO), musculoskeletal disorders are the second largest contributor to disability worldwide. The increasing prevalence of musculoskeletal disorders has created a significant demand for advanced therapies such as cell & gene therapy, driving the growth of the market.
- Growing Investment in Research and Development helps to drive the market growth: -
- Another major driver for the cell and gene therapy for musculoskeletal diseases market is the growing investment in research and development activities by biotech & pharmaceutical companies. Many companies are investing heavily in the development of novel therapies to treat musculoskeletal disorders. For instance, in 2020, Regeneron Pharmaceuticals and Intellia Therapeutics collaborated to develop CRISPR/Cas gene editing technology for the treatment of musculoskeletal diseases. Such investments are projected to accelerate the development of new and innovative cell and gene therapies, driving the growth of the market.
Market Restraints:
- The Cell and Gene Therapy for Musculoskeletal Diseases Market's growth is being stifled by Stringent Regulatory Frameworks:
- One of the major restraints for the cell & gene therapy for musculoskeletal diseases market is the stringent regulatory framework for the approval of these therapies. The regulatory approval process involves several stages of clinical trials, which increases the cost & time required for market entry.
- The Cell and Gene Therapy for Musculoskeletal Diseases Market's growth is being stifled by High Cost of Therapies:
- The high cost of cell and gene therapies for musculoskeletal diseases is another momentous restraint for the market. These therapies require complex manufacturing processes, which increase their production cost. Additionally, the high cost of clinical trials and the limited availability of these therapies also contribute to the high pricing, making it inaccessible to a momentous population. This high cost also hinders the acceptance of these therapies in developing countries with limited healthcare budgets.
CELL AND GENE THERAPY FOR MUSCULOSKELETAL DISEASES MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2022 - 2030 |
|
Base Year |
2022 |
|
Forecast Period |
2023 - 2030 |
|
CAGR |
7.8 % |
|
Segments Covered |
By Gene Type, Disease Type, Vector Type, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Mesoblast Ltd., TiGenix NV, Vericel Corporation, Osiris Therapeutics, Inc., Stryker Corporation, Zimmer Biomet Holdings, Inc., Medipost Co., Ltd., RTI Surgical Holdings, Inc., CollPlant Biotechnologies Ltd., Orthocell Ltd. |
Market Segments:
This research report on the global Cell and Gene Therapy for Musculoskeletal Diseases Market has been segmented based on Disease Type, Gene Type, Vector Type and Region.
Cell and Gene Therapy for Musculoskeletal Diseases Market—By Disease type
- Osteoarthritis
- Rheumatoid Arthritis
- Bone Fracture
- Osteoporosis
- Others
The market for gene therapy for musculoskeletal diseases can be segmented based on disease type into osteoarthritis, rheumatoid arthritis, bone fracture, osteoporosis, and others. Osteoarthritis holds the largest market share due to the high prevalence of the disease and the increasing geriatric population. The rise in demand for advanced therapeutics and personalized medicines has also driven the market for gene therapy for osteoarthritis. The rheumatoid arthritis segment is also projected to witness significant growth due to the high unmet medical needs and the increasing research and development activities for the development of advanced therapies. The bone fracture segment is projected to grow due to the increasing prevalence of bone fractures in the aging population and the need for faster healing and recovery. The osteoporosis segment is projected to witness significant growth due to the high prevalence of the disease & the increasing awareness regarding the disease and its management. The "others" segment includes various rare musculoskeletal disorders that can be targeted through gene therapy, such as muscular dystrophy and spinal muscular atrophy, which are also projected to contribute to the market growth.
Cell and Gene Therapy for Musculoskeletal Diseases Market – By Gene Type
- Bone Morphogenetic Protein (BMP)
- Fibroblast Growth Factor (FGF)
- Transforming Growth Factor-Beta (TGF-β)
- Platelet-Derived Growth Factor (PDGF)
- Others
The gene therapy for musculoskeletal diseases market is also segmented based on the gene types, including bone morphogenetic protein (BMP), fibroblast growth factor (FGF), transforming growth factor-beta (TGF-β), platelet-derived growth factor (PDGF), and others. BMP is projected to dominate the market due to its therapeutic potential in bone regeneration and repair, as it stimulates the production of bone cells and has been approved by the FDA for use in spinal fusion surgeries. Additionally, FGF and TGF-β are also anticipated to have a significant market share, owing to their ability to induce bone formation and repair damaged tissue. The PDGF segment is projected to show a lucrative growth rate in the coming years, as it has shown promising results in enhancing bone regeneration and healing in clinical trials. Overall, the growing research and development activities for gene therapy for musculoskeletal diseases & the increasing prevalence of such diseases are projected to drive the market growth across all gene types.
Cell and Gene Therapy for Musculoskeletal Diseases Market – By Vector Type
- Retroviral Vector
- Adenoviral Vector
- Adeno-Associated Viral Vector
- Lentiviral Vector
- Plasmid DNA Vector
- Others
The gene therapy for musculoskeletal diseases market can also be segmented based on vector type, which includes retroviral vector, adenoviral vector, adeno-associated viral vector, lentiviral vector, plasmid DNA vector, and others. Among these, adeno-associated viral vectors are projected to have the largest market share due to their higher transduction efficiency and safety profile. Lentiviral vectors are also gaining popularity in gene therapy due to their ability to deliver the therapeutic gene to both dividing and non-dividing cells. Moreover, plasmid DNA vectors are considered to be a safe and easy-to-produce option for gene therapy, and are being investigated in clinical trials for the treatment of musculoskeletal diseases. The choice of vector type depends on various factors such as the target cell type, duration of gene expression required, and safety profile of the vector.
Cell and Gene Therapy for Musculoskeletal Diseases Market – By Region
- North America
- Europe
- Asia-Pacific
- Rest of the World
The gene therapy for musculoskeletal diseases market is segmented based on region into North America, Europe, Asia Pacific, & Rest of the World. North America is projected to hold the largest market share, owing to the presence of established healthcare infrastructure, high investments in R&D activities, and favourable government initiatives. Europe is also projected to witness momentous growth, driven by the rising prevalence of musculoskeletal disorders and the increasing adoption of advanced therapies. The Asia Pacific region is projected to exhibit the highest growth rate, owing to the increasing prevalence of musculoskeletal diseases, rising healthcare expenditure, and increasing government initiatives to promote gene therapies. In addition, the region has a large patient pool and a high demand for advanced healthcare solutions, thereby creating significant growth opportunities. The rest of the world segment is also projected to witness steady growth, driven by the increasing prevalence of musculoskeletal diseases and the growing focus on improving healthcare infrastructure.
Major Key Players in the Market:
- Mesoblast Ltd.
- TiGenix NV
- Vericel Corporation
- Osiris Therapeutics, Inc.
- Stryker Corporation
- Zimmer Biomet Holdings, Inc.
- Medipost Co., Ltd.
- RTI Surgical Holdings, Inc.
- CollPlant Biotechnologies Ltd.
- Orthocell Ltd.
Market Insights and Developments:
- In January 2021, the US FDA approved Novartis's Kymriah, a CAR-T cell therapy for the treatment of relapsed or refractory diffuse large B-cell lymphoma, expanding its indication beyond the previously sanctioned pediatric acute lymphoblastic leukemia. This approval represents a significant milestone in the development of cell-based therapies, as Kymriah is the first gene therapy to receive FDA approval.
- In May 2020, the US-based biotech company Amgen announced positive results from a Phase 1/2 clinical trial of AMG 509, a small molecule inhibitor for the treatment of osteoarthritis. The drug was shown to reduce joint pain & improve function, offering a potential new treatment option for the estimated 27 million people in the US alone who suffer from osteoarthritis.
- In March 2020, a group of researchers from the UK's University of Sheffield published a study demonstrating the potential of gene therapy to regenerate damaged cartilage in joints. Using a CRISPR/Cas9 gene editing technique, the researchers were able to "reprogram" stem cells to become chondrocytes, the cells responsible for producing cartilage. The study's findings could pave the way for new treatments for conditions such as osteoarthritis and rheumatoid arthritis.
Chapter 1. CELL AND GENE THERAPY FOR MUSCULOSKELETAL DISEASES MARKET – Scope & Methodology
1.1. Market Segmentation
1.2. Assumptions
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2. CELL AND GENE THERAPY FOR MUSCULOSKELETAL DISEASES MARKET – Executive Summary
2.1. Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-19 Impact Analysis
2.3.1. Impact during 2023 – 2030
2.3.2. Impact on Supply – Demand
Chapter 3. CELL AND GENE THERAPY FOR MUSCULOSKELETAL DISEASES MARKET – Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4. CELL AND GENE THERAPY FOR MUSCULOSKELETAL DISEASES MARKET - Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatory Scenario - By Region
4.3 Customer Analysis
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. CELL AND GENE THERAPY FOR MUSCULOSKELETAL DISEASES MARKET - Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6. CELL AND GENE THERAPY FOR MUSCULOSKELETAL DISEASES MARKET – by Disease type
6.1. Osteoarthritis
6.2. Rheumatoid Arthritis
6.3. Bone Fracture
6.4. Osteoporosis
6.5. Others
Chapter 7. CELL AND GENE THERAPY FOR MUSCULOSKELETAL DISEASES MARKET – By Gene Type
7.1. Bone Morphogenetic Protein (BMP)
7.2. Fibroblast Growth Factor (FGF)
7.3. Transforming Growth Factor-Beta (TGF-β)
7.4. Platelet-Derived Growth Factor (PDGF)
7.5. Others
Chapter 8. CELL AND GENE THERAPY FOR MUSCULOSKELETAL DISEASES MARKET – By Vector Type
8.1. Retroviral Vector
8.2. Adenoviral Vector
8.3. Adeno-Associated Viral Vector
8.4. Lentiviral Vector
8.5. Plasmid DNA Vector
8.6. Others
Chapter 9. CELL AND GENE THERAPY FOR MUSCULOSKELETAL DISEASES MARKET – By Region
9.1. North America
9.2. Europe
9.3.The Asia Pacific
9.4.Latin America
9.5. Middle-East and Africa
Chapter 10. CELL AND GENE THERAPY FOR MUSCULOSKELETAL DISEASES MARKET– Company Profiles – (Overview, Product Portfolio, Financials, Developments)
10.1. Mesoblast Ltd.
10.2. TiGenix NV
10.3. Vericel Corporation
10.4. Osiris Therapeutics, Inc.
10.5. Stryker Corporation
10.6. Zimmer Biomet Holdings, Inc.
10.7. Medipost Co., Ltd.
10.8. RTI Surgical Holdings, Inc.
10.9. CollPlant Biotechnologies Ltd.
10.10. Orthocell Ltd.
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
Cell and gene therapy for musculoskeletal diseases is a new approach to treating these conditions using cellular and genetic materials to repair or replace damaged tissues and cells
Cell and gene therapy is currently being researched for a range of musculoskeletal diseases, including osteoarthritis, rheumatoid arthritis, muscular dystrophy, and spinal cord injuries
The potential benefits of cell and gene therapy for musculoskeletal diseases include improved pain relief, reduced inflammation, enhanced tissue repair, and the potential for a long-term cure.
Like any medical procedure, there are risks associated with cell and gene therapy for musculoskeletal diseases. These can include infections, immune reactions, and other complications.
While research into cell and gene therapy for musculoskeletal diseases is ongoing, these treatments are still in the early stages of development and are not widely available to patients at this time. However, as research progresses, it is possible that these therapies will become more widely available in the future.