CAR T cell Therapy Market Size (2024 – 2030)
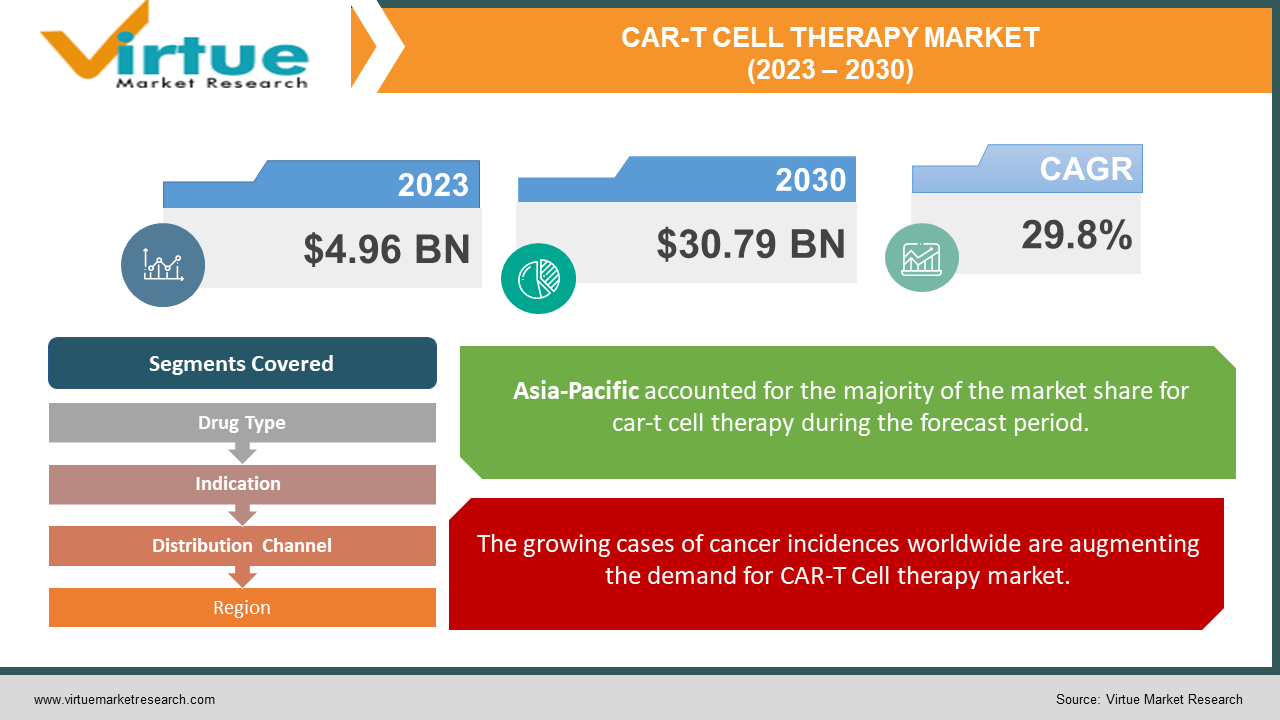
The CAR-T Cell Therapy Market was valued at USD 4.96 billion in 2023 and is projected to reach a market size of USD 30.79 billion by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 29.8%.
Key Market Insights:
27% of the overall T-cell therapy market is accounted for by CAR T-cell therapy. CAR T-Cells are expected to be used for therapeutic applications due to the rising incidence of cancer. Synthetic T-cell receptors can be used to treat various cancers.
T-cells can find and kill cancer cells with the help of chimeric immunoreceptors. There is continued research and development in life science and biotechnology for the treatment of cancer. In various countries, studies are being conducted to confirm the efficacy of CAR T-Cell therapy, which will aid in improving data availability regarding efficacy, mechanism of action, and compliance in patients with leukemia and lymphoma.
Increasing approvals and expanding indications. CAR-T cell therapies have shown great success in treating leukemia and other hematological malignancies. Increasing regulatory approvals for new indications and expanding its use to treat a broader range of cancers are included in the outlook for CAR-T cell therapy. The efficacy and safety of CAR-T cell therapy in various cancer types are being explored in ongoing clinical trials.
There are advances in technology and manufacturing. Significant improvements in CAR-T cell therapy technology are expected.
There are applications for children. Children with relapsed or refractory ALL have shown significant efficacy with CAR-T cell therapy. Expanding the use of CAR-T cell therapy to treat other children's cancers is part of the outlook for the therapy.
There are ongoing research and clinical trials that are focused on improving CAR-T cell therapy for children.
CAR-T Cell Therapy Market Drivers:
The growing cases of cancer incidences worldwide are augmenting the demand for CAR-T Cell therapy market.
The CAR-T cell therapy market is growing due to increasing patient assistance programs, growing government measures to raise cancer awareness, and robust R&D investments from major companies. The demand for cell-based therapy is growing.
The expansion of the global CAR T-Cell treatment market is expected to be spurred by an increase in cancer incidence. Cancer is the leading cause of death. Most cancer cases are caused by harmful lifestyle factors. Medical technology and personalized medicine have improved.
The CAR T-Cell therapy products have a robust product line. AUTO1, an innovative product from Autolus, is undergoing a phase I trial to treat adult acute lymphoblastic leukemia. There are clinical safety and activity limitations associated with existing CD19 CAR T-Cell therapies.
There is a growing demand for personalized and targeted therapies. Chemo and radiation therapy are non-specific and can damage healthy cells as well as cancer cells. This can lead to serious side effects and a reduced quality of life. CAR-T cell therapy is designed to attack cancer cells without harming healthy cells. CAR-T cell therapy is becoming more and more attractive for patients with cancer.
There has been an increase in the approval of novel medications driving market growth.
Increased awareness of CAR-T cells and the development of new technology creates armored CARs that express pro-inflammatory cytokine like IL-12 or IL-15. CAR T-Cell proliferation and persistence increase at the beginning of the immunosuppression.
Investments in research and development of CAR-T cell therapies are increasing:
CAR-T cell therapy is effective in the treatment of cancer. Increased investment in research and development of CAR-T cell therapies is a result of this. Many companies are investing in the development of CAR-T cell therapies.
The CAR-T Cell Therapy Market is growing due to the increasing governmental investment.
Increased investment in medical infrastructure leads to better facilities and equipment that can facilitate the development and production of CAR-T cell therapies. Increased investment in medical maintenance ensures that these facilities and equipment are up-to-date and functioning properly, which is essential for the safe and effective administration of CAR-T cell therapies. Improved diagnosis and treatment options, expanded access to care, enhanced patient support systems, increased public awareness, and the promotion of precision medicine are some of the positive outcomes of these investments. As a result of these factors, the CAR-T Cell Therapy market is expected to continue to grow, with new and more effective treatments being developed to combat this challenging disease.
CAR-T Cell Therapy Market Restraints and Challenges:
The high cost of treatment is one of the major constraints of the CAR-T cell therapy market. The cost of manufacturing and administering CAR-T cell therapy can be very high. CAR-T cell therapy can cost as much as $400,000 per treatment in the United States. The demand for CAR-T Cell Therapy diagnosis and treatment can be affected by this. Conventional CAR-T Cell Therapy treatment may not be the best option for some patients. This can affect the growth of the global CAR-T Cell Therapy market. Additionally, there are safety concerns associated with CAR-T cell therapy. CAR-T cell therapy can cause several serious side effects. CRS is a potentially life-threatening condition that can cause a wide range of symptoms. Neurological symptoms can be caused by toxicity. TLS can cause problems with the body.
CAR-T CELL THERAPY MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
29.8% |
|
Segments Covered |
By Drug Type, Indication, Distribution Channel and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Pfizer, Inc., Novartis Ag, Bristol-Myers Squibb, Amgen, Inc., Sorrento Therapeutics, Inc., Johnson & Johnson Services, Inc., Gilead Sciences, Inc., Merck & Co., Inc., bluebird bio, Inc. |
CAR-T Cell Therapy Market Segmentation: By Drug Type
-
Axicabtagene Ciloleucel
-
Tisagenlecleucel
-
Brexucabtagene Autoleucel
-
Others
The global CAR T-Cell therapy market was dominated by axicabtagene ciloleucel, and this trend is predicted to continue throughout the forecasted years. Yescarta contains the active ingredient axicabtagene ciloleucel. The need for Yescarta is expected to drive segment growth during the estimated period. tisagenlecleucel is expected to be the fastest-growing segment between 2024 and 2030. The segment expansion is due to an increased need for Kymriah in various countries.
CAR-T Cell Therapy Market Segmentation: By Indication
-
Lymphoma
-
Acute Lymphocytic Leukemia
- Others
B-cell lymphoma, leukemia, solid tumors, multiple myeloma, and other indications are included in the CAR-T Cell Therapy Market. The B-cell lymphoma segment has the highest revenue share. The segment is experiencing high growth due to the increasing prevalence of B-cell lymphoma and the growing adoption of CAR-T cell therapy for the treatment of this indication. The Leukemia segment is expected to grow at a CAGR of over 15% during the forecast period.
The growth of this segment can be attributed to the increasing adoption of CAR-T cell therapy for the treatment of leukemia and the growing awareness of the benefits of CAR-T cell therapy in this indication. Solid tumors are the third largest segment and are expected to grow at a CAGR of over 10% during the forecast period. The growth of this segment is being driven by the increasing prevalence of solid tumors and the growing number of clinical trials evaluating the efficacy of CAR-T cell therapy in the treatment of solid tumors.
Multiple myeloma is the fourth largest segment and is expected to grow at a CAGR of over 5% during the forecast period.
The increasing prevalence of multiple myeloma and the growing adoption of CAR-T cell therapy for the treatment of this indication are driving the growth of this segment.
Other indications are expected to grow at a CAGR of over 5%. An increasing number of clinical trials evaluating the efficacy of CAR-T cell therapy in the treatment of other indications is driving the growth of this segment.
CAR-T Cell Therapy Market Segmentation: By Distribution Channel
-
Hospitals
-
Cancer Treatment Centers
-
Pharmacy
Hospitals account for 55% of the market share in 2023. Clinics and ambulatory surgical centers have 25% of the market. Pharmacy chains and online pharmacies make up 20% of the market devoted to clinics for CAR-T Cell Therapy care. Clinics can provide a range of CAR-T Cell Therapy services, including diagnostic testing, screening, and treatment, and they are often more convenient and affordable than hospitals. 10% of the market for CAR-T Cell Therapy drugs is supplied by pharmacy chains. Pharmacy chains offer a convenient way for patients to fill their prescriptions, and they can provide counselling and support to patients.
CAR-T Cell Therapy Market Segmentation: Regional Analysis:
-
North America
-
Asia-Pacific
-
Europe
-
South America
-
Middle East and Africa
North American region dominated the CAR-T Cell Therapy Market with a revenue of 39%. The region has a well-developed healthcare infrastructure and advanced medical facilities, which allow early diagnosis and treatment of breast cancer. Increased awareness among patients has led to increased screening and detection rates. Many pharmaceutical companies, research institutions, and academic centres are located in North America.
Europe has a market share of over 25% for CAR-T cell therapy. The market is expected to grow due to the increasing prevalence of cancer in the region, the growing demand for personalized and targeted therapies, and the increasing investments in research and development of CAR-T cell therapies. The market is expected to grow due to the increasing prevalence of cancer in the region, the growing demand for personalized and targeted therapies, and the increasing investments in research and development of CAR-T cell therapies.
Asia-Pacific region is the fastest growing region and has plenty number of opportunities due to an increase in the cases of cancer and an increase in demand for targeted therapies and government investment in the R&D of CAR-T cell therapies.
COVID-19 Impact Analysis on the CAR-T Cell Therapy Market:
CAR T-Cell therapy has been impacted by the coronaviruses. Supply networks for critical medicines were disrupted by the decline in the transportation of human organs. The market for CAR T-Cell treatment is expected to grow more slowly as a result of the ongoing Pandemic.
Patients with relapsed/refractory hematologic malignancies are the most affected by this situation. Telemedicine encourages early intervention of tocilizumab-based neurotoxicity or cytokine release syndrome.
Latest Trends/ Developments:
CAR-T cell therapies are new. Some of the limitations of current CAR-T cell therapies are being overcome by next-generation CAR-T cell therapies. The therapies include:
CAR-T cell therapies have dual targets. The therapies target two different antigens on the cancer cell, which can help to prevent the cancer cells from escaping the therapy.
CAR-T cell therapies are universal. The use of genetically modified T cells from a healthy donor can eliminate the need for patients to undergo leukapheresis. CAR-T cell therapies are allogeneic. These therapies use T cells from a healthy donor, which can be used to treat patients with incompatible cancer cells.
Key Players:
-
Pfizer, Inc.
-
Novartis Ag
-
Bristol-Myers Squibb
-
Amgen, Inc.
-
Sorrento Therapeutics, Inc.
-
Johnson & Johnson Services, Inc.
-
Gilead Sciences, Inc.
-
Merck & Co., Inc.
-
bluebird bio, Inc.
In September 2020, Seattle Genetics, Inc. Two new strategic cancer studies were announced. There is a wide-ranging joint development program conducted by the partnership. Keytruda is approved for the treatment of melanoma patients with involvement in the lystium.
In December 2020, Atara Biotherapeutics, Inc. and Bayer AG declared. The two companies will work together to develop CAR T-Cell therapies for solid tumors. According to the agreement, the company is concentrating on developing a treatment for lung cancer. The company was able to become the leading manufacturer of allogeneic CAR-T cell therapy because of this collaboration.
Chapter 1. CAR-T Cell Therapy Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. CAR-T Cell Therapy Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. CAR-T Cell Therapy Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. CAR-T Cell Therapy Market Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. CAR-T Cell Therapy Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. CAR-T Cell Therapy Market – By Drug Type
6.1 Introduction/Key Findings
6.2 Axicabtagene Ciloleucel
6.3 Tisagenlecleucel
6.4 Brexucabtagene Autoleucel
6.5 Others
6.6 Y-O-Y Growth trend Analysis By Drug Type
6.7 Absolute $ Opportunity Analysis By Drug Type, 2024-2030
Chapter 7. CAR-T Cell Therapy Market – By Indication
7.1 Introduction/Key Findings
7.2 Lymphoma
7.3 Acute Lymphocytic Leukemia
7.4 Others
7.5 Y-O-Y Growth trend Analysis By Indication
7.6 Absolute $ Opportunity Analysis By Indication, 2024-2030
Chapter 8. CAR-T Cell Therapy Market – By Distribution Channel
8.1 Introduction/Key Findings
8.2 Hospitals
8.3 Cancer Treatment Centers
8.4 Pharmacy
8.5 Y-O-Y Growth trend Analysis By Distribution Channel
8.6 Absolute $ Opportunity Analysis By Distribution Channel, 2024-2030
Chapter 9. CAR-T Cell Therapy Market , By Geography – Market Size, Forecast, Trends & Insights
9.1 North America
9.1.1 By Country
9.1.1.1 U.S.A.
9.1.1.2 Canada
9.1.1.3 Mexico
9.1.2 By Drug Type
9.1.3 By Indication
9.1.4 By By Distribution Channel
9.1.5 Countries & Segments - Market Attractiveness Analysis
9.2 Europe
9.2.1 By Country
9.2.1.1 U.K
9.2.1.2 Germany
9.2.1.3 France
9.2.1.4 Italy
9.2.1.5 Spain
9.2.1.6 Rest of Europe
9.2.2 By Drug Type
9.2.3 By Indication
9.2.4 By Distribution Channel
9.2.5 Countries & Segments - Market Attractiveness Analysis
9.3 Asia Pacific
9.3.1 By Country
9.3.1.1 China
9.3.1.2 Japan
9.3.1.3 South Korea
9.3.1.4 India
9.3.1.5 Australia & New Zealand
9.3.1.6 Rest of Asia-Pacific
9.3.2 By Drug Type
9.3.3 By Indication
9.3.4 By Distribution Channel
9.3.5 Countries & Segments - Market Attractiveness Analysis
9.4 South America
9.4.1 By Country
9.4.1.1 Brazil
9.4.1.2 Argentina
9.4.1.3 Colombia
9.4.1.4 Chile
9.4.1.5 Rest of South America
9.4.2 By Drug Type
9.4.3 By Indication
9.4.4 By Distribution Channel
9.4.5 Countries & Segments - Market Attractiveness Analysis
9.5 Middle East & Africa
9.5.1 By Country
9.5.1.1 United Arab Emirates (UAE)
9.5.1.2 Saudi Arabia
9.5.1.3 Qatar
9.5.1.4 Israel
9.5.1.5 South Africa
9.5.1.6 Nigeria
9.5.1.7 Kenya
9.5.1.8 Egypt
9.5.1.9 Rest of MEA
9.5.2 By Drug Type
9.5.3 By Indication
9.5.4 By Distribution Channel
9.5.5 Countries & Segments - Market Attractiveness Analysis
Chapter 10. CAR-T Cell Therapy Market – Company Profiles – (Overview, Drug Type Portfolio, Financials, Strategies & Developments)
10.1 Novozymes (Denmark)
10.2 DSM (Netherlands)
10.3 Chr. Hansen (Denmark)
10.4 Amano Enzymes (Japan)
10.5 Associated British Foods (UK)
10.6 DowDuPont (US)
10.7 Advanced Enzymes (India)
10.8 Enzyme Development Corporation (US)
10.9 Aumgene Biosciences (India)
10.10 Biocatalysts (UK)
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The key trends in the CAR-T Cell Therapy Drugs Market are an increase in the aging population of women, a rise in the prevalence of cancer, the launch of innovative medications, a boost in healthcare expenditure, and higher government support
An increasing number of CAR-T cell therapy product approvals, an increase in cancer cases, and increased demand for CAR-T cell therapy supplies are some of the driving factors of the CAR T-cell therapy market.
Based on Indication Type, the CAR-T Cell Therapy Market is segmented into Lymphoma, Acute Lymphocytic Leukemia, and Others.
North America is the most dominant region for the CAR-T Cell Therapy Market
Pfizer, Inc., Novartis Ag, Bristol-Myers Squibb, Amgen, Inc., Sorrento Therapeutics, Inc., Johnson & Johnson Services, Inc., Gilead Sciences, Inc., Merck & Co., Inc., Bluebird bio, Inc.



