Cancer Genetic Biomarkers For Neuroendocrine Tumor Market Size (2024 – 2030)
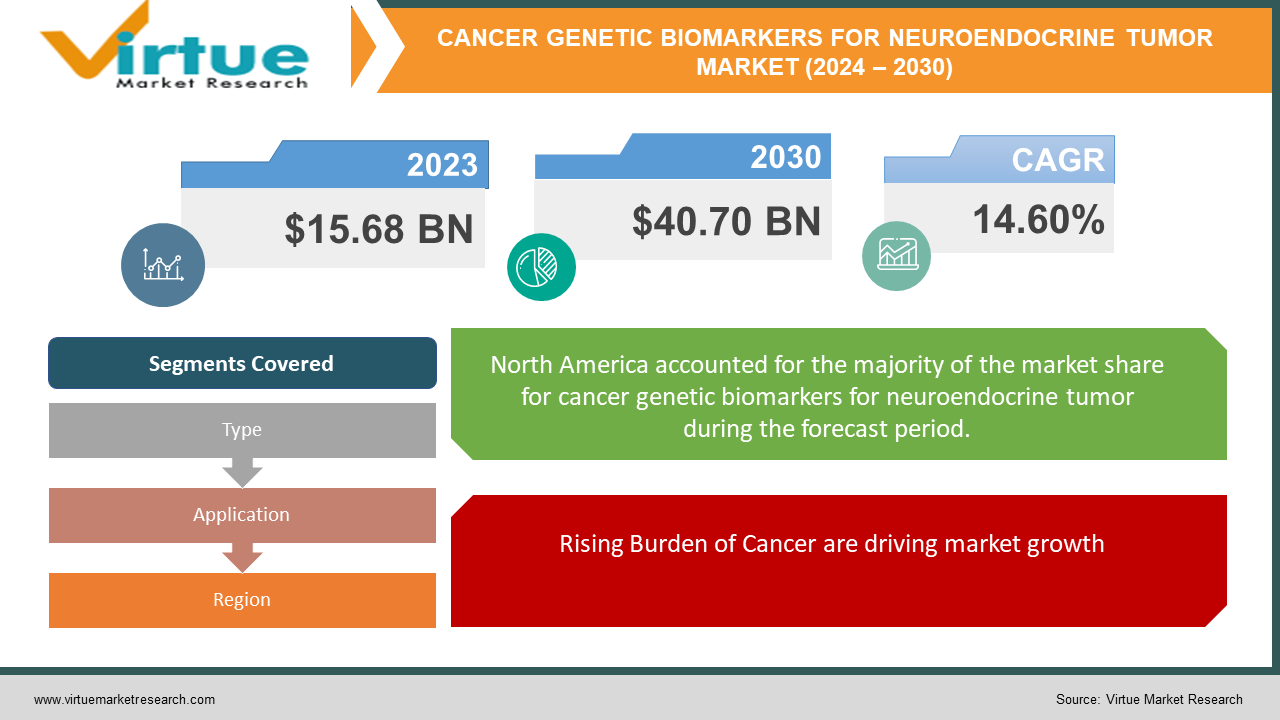
The Global Cancer Genetic Biomarkers For Neuroendocrine Tumor Market was valued at USD 15.68 billion in 2023 and will grow at a CAGR of 14.60% from 2024 to 2030. The market is expected to reach USD 40.70 billion by 2030.
The Cancer Genetic Biomarkers for Neuroendocrine Tumor Market focuses on identifying specific genetic markers in tumors to improve diagnosis, predict disease course, and personalize treatment for patients with neuroendocrine tumors. This niche market is expected to grow alongside the rising need for better detection and targeted therapies in cancer, but specific market size data isn't yet widely available.
Key Market Insights:
The global burden of cancer is anticipated to rise significantly, propelling the demand for advanced diagnostic tools like genetic biomarkers. The growing focus on developing targeted therapies and personalized treatment plans for cancer patients is a major driver for this market.
Genetic biomarkers can aid in earlier and more accurate diagnosis of neuroendocrine tumors, leading to better patient outcomes.
Currently, there might be a limited number of commercially available genetic tests for neuroendocrine tumors.
Reimbursement policies for these tests by insurance companies might be unclear or restrictive.
Global Cancer Genetic Biomarkers For Neuroendocrine Tumor Market Drivers:
Rising Burden of Cancer are driving market growth:
A looming shadow is cast over the future of healthcare as the global incidence of cancer is expected to rise dramatically. This surge is attributed to a confluence of factors: aging populations, where the natural risk of cancer increases with age, and concerning lifestyle trends like smoking and unhealthy diets. To combat this escalating challenge, the demand for advanced diagnostic tools will surge, particularly in the realm of genetic biomarkers for neuroendocrine tumors. These specialized markers have the potential to revolutionize how we diagnose and treat these tumors. By analyzing a patient's genetic makeup, doctors can gain crucial insights into the specific type of tumor present, its potential for aggressiveness, and even how a patient might respond to different therapies. This information empowers physicians to tailor treatment plans to the unique biology of each tumor, maximizing effectiveness while minimizing side effects. This targeted approach holds immense promise for improving patient outcomes and underscores the critical role genetic biomarkers will play in the future of neuroendocrine tumor diagnosis and treatment.
Increased Focus on Personalized Medicine are driving market growth:
The era of a single treatment for all cancers is fading as personalized medicine ushers in a new era of precision. This paradigm shift moves away from a "one-size-fits-all" approach and instead tailors treatments to the specific genetic makeup of a patient's tumor. Genetic biomarkers are the cornerstone of this revolution, acting as unique fingerprints that reveal the underlying biology of the cancer. By analyzing these biomarkers, doctors can identify the specific mutations driving the tumor's growth. This newfound knowledge unlocks the door to the development of targeted therapies – drugs designed to exploit these very mutations. These targeted therapies offer a beacon of hope, with the potential for superior efficacy compared to traditional treatments. Additionally, they can come with a reduced side-effect profile, as they specifically target the cancer cells and minimize damage to healthy tissues. This personalized approach holds immense promise for improved patient outcomes. Imagine a future where treatment plans are crafted to an individual's unique cancer, maximizing effectiveness while minimizing the burden on the patient's body. Genetic biomarkers are the key to unlocking this future, making them a game-changer in the fight against cancer.
Improved Diagnosis and Early Detection are driving market growth:
In the fight against neuroendocrine tumors, early and accurate diagnosis is the cornerstone of successful treatment. Here, genetic biomarkers emerge as powerful allies. Traditional diagnostic methods like imaging and biopsies can sometimes be ambiguous. Genetic biomarkers offer a more precise and objective approach. By analyzing a patient's unique genetic fingerprint, these markers can help differentiate between various tumor types, a crucial step in guiding treatment decisions. Furthermore, they can identify patients at a higher risk of recurrence. This allows physicians to implement preventative measures and closer monitoring, potentially catching any regrowth early and improving long-term outcomes. But the power of genetic biomarkers goes beyond diagnosis. By revealing the tumor's underlying genetic makeup, they can predict how a patient might respond to specific therapies. This predictive power empowers doctors to tailor treatment plans, prioritizing medications with the highest chance of success and minimizing the use of ineffective drugs. This personalized approach not only improves treatment effectiveness but also reduces unnecessary side effects, significantly improving a patient's quality of life throughout the treatment journey. In essence, genetic biomarkers offer a window into the unique biology of each neuroendocrine tumor, paving the way for a new era of precise diagnosis and targeted treatment.
Global Cancer Genetic Biomarkers For Neuroendocrine Tumor Market challenges and restraints:
Limited Availability of Tests is a significant hurdle for Cancer Genetic Biomarkers For Neuroendocrine Tumor:
A major hurdle in the Global Cancer Genetic Biomarkers for Neuroendocrine Tumor Market is the limited availability of commercial tests. Unlike some other cancers, there may only be a handful of genetic tests currently available that are specifically designed to diagnose and characterize neuroendocrine tumors. This restricted menu creates a bottleneck for patients seeking this advanced diagnostic tool. Imagine a scenario where a doctor suspects a patient has a neuroendocrine tumor, but the confirmatory genetic test isn't readily available. This lack of options can lead to delays in diagnosis, which can be detrimental in cancers where early intervention is critical. Furthermore, the limited variety of tests might not capture the full spectrum of genetic alterations present in neuroendocrine tumors. This could hinder the effectiveness of the tests in providing a comprehensive picture of the disease, potentially leading to missed diagnoses or inaccurate treatment plans. Expanding the range of commercially available genetic tests is crucial to bridge this gap and ensure that patients have access to the most advanced diagnostic tools for accurate and timely diagnosis of neuroendocrine tumors.
Reimbursement Issues are throwing a curveball at Cancer Genetic Biomarkers For Neuroendocrine Tumor market:
A significant barrier to wider adoption of genetic testing for neuroendocrine tumors lies in the murky world of insurance coverage. Unlike some established diagnostic procedures, the coverage for these tests by insurance companies can be unclear or severely limited. This translates into a hefty financial burden for patients considering this advanced diagnostic approach. Imagine a patient facing a potential neuroendocrine tumor diagnosis. Genetic testing could offer valuable insights for guiding treatment, yet the upfront cost might be substantial and not fully covered by insurance. This financial uncertainty can be a major deterrent, potentially forcing patients to choose less-precise diagnostic methods or even forgo testing altogether. The lack of clear insurance coverage creates a double-edged sword. It discourages patients from seeking potentially life-saving information while simultaneously limiting the data available to healthcare providers on the effectiveness of these tests. Clearer and more comprehensive insurance coverage for genetic testing is crucial to ensure equitable access to this advanced diagnostic tool and to facilitate the collection of valuable data that can further refine and improve the use of genetic biomarkers in the fight against neuroendocrine tumors.
High Cost of Developing and Implementing Tests are a growing nightmare for Cancer Genetic Biomarkers For Neuroendocrine Tumor:
High costs pose a double challenge in the Global Cancer Genetic Biomarkers for Neuroendocrine Tumor Market. Firstly, the research and development (R&D) of these tests is inherently expensive. Developing new genetic biomarkers requires sophisticated technologies like DNA sequencing and bioinformatics analysis. These high-tech processes necessitate significant financial investment from research institutions and pharmaceutical companies. This translates to a limited number of companies actively pursuing the development of new tests, potentially hindering innovation in the field. Secondly, even after development, implementing these tests in clinical settings adds another layer of cost. These tests often require specialized equipment and highly trained personnel to perform and interpret the results accurately. This specialized infrastructure might not be readily available in all healthcare facilities, creating a situation where even if a test exists, access to it might be limited. To bridge this gap, innovative solutions are needed to bring down the R&D costs and streamline the implementation process. This could involve increased collaboration between research institutions and diagnostic companies, as well as the development of user-friendly test kits that can be used in a wider range of clinical settings.
Market Opportunities:
The Cancer Genetic Biomarkers for Neuroendocrine Tumor Market presents exciting opportunities fueled by rising needs and advancements in the field. The escalating global cancer burden, particularly the projected increase in neuroendocrine tumors, creates a strong demand for more effective diagnostic tools. Genetic biomarkers offer a promising solution with their ability to provide earlier, more accurate diagnoses and personalized treatment plans. This shift towards precision medicine, where treatments are tailored to a patient's unique tumor biology, opens a significant avenue for growth. Furthermore, advancements in DNA sequencing technologies and bioinformatics are paving the way for the development of more specific and reliable genetic tests. This will likely lead to the identification of novel biomarkers specific to different subtypes of neuroendocrine tumors, allowing for even more targeted diagnoses and treatment strategies. Additionally, growing awareness about the benefits of genetic testing in cancer diagnosis is fostering a supportive environment for market expansion. This is further fueled by increasing research activities and collaborations between research institutions, pharmaceutical companies, and clinical diagnostic labs. These collaborations hold immense potential to accelerate innovation, improve test affordability, and ensure wider accessibility for patients. However, overcoming challenges like limited test availability, unclear insurance coverage, and high costs associated with R&D and implementation will be crucial for unlocking the full potential of this market. By addressing these hurdles and capitalizing on the existing opportunities, the Cancer Genetic Biomarkers for Neuroendocrine Tumor Market is poised for significant growth in the years to come.
CANCER GENETIC BIOMARKERS FOR NEUROENDOCRINE TUMOR MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
14.60% |
|
Segments Covered |
By Type, Application, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
F. Hoffmann-La Roche Ltd., Abbott Laboratories, Illumina, Inc., QIAGEN N.V., Thermo Fisher Scientific Inc., Myriad Genetics, Inc., Roche Diagnostics, Agilent Technologies Inc., Merck KGaA, Darmstadt, Germany, Invitae Corporation |
Cancer Genetic Biomarkers For Neuroendocrine Tumor Market segmentation - By Type
-
Mutation biomarkers
-
Gene expression biomarkers
Currently, mutation biomarkers hold the leading role in the Cancer Genetic Biomarkers for Neuroendocrine Tumor Market. These markers target specific gene mutations known to drive neuroendocrine tumor development. Their ability to definitively identify tumor types and guide treatment decisions makes them a valuable tool for clinicians. However, gene expression biomarkers are emerging as a promising challenger. By analyzing the overall gene activity within a tumor, they offer a broader picture of the biological processes at play. This deeper understanding could lead to the identification of novel therapeutic targets and even more personalized treatment strategies. As research progresses, gene expression biomarkers have the potential to become a significant force in the market, but for now, mutation biomarkers remain the dominant player.
Cancer Genetic Biomarkers For Neuroendocrine Tumor Market segmentation - By Application
-
Diagnosis
-
Prognosis
Diagnosis currently holds the most prominent position within the Cancer Genetic Biomarkers for Neuroendocrine Tumor Market. Accurate and early diagnosis is critical for successful treatment in these cancers. Genetic biomarkers excel in this area by offering a more precise and objective method compared to traditional diagnostic tools like imaging or biopsies. They can differentiate between various tumor types, ensuring patients receive the most appropriate treatment plan from the outset. While prognosis using genetic biomarkers is gaining traction, it's still a developing field. However, the ability to predict a tumor's aggressiveness and recurrence risk holds immense promise for personalizing treatment plans and improving patient outcomes. As research advances, the role of genetic biomarkers in prognosis is likely to become more prominent, but for now, diagnosis reigns supreme in this market.
Cancer Genetic Biomarkers For Neuroendocrine Tumor Market segmentation - Regional Analysis
-
North America
-
Asia-Pacific
-
Europe
-
South America
-
Middle East and Africa
Currently, North America is likely the most dominant region in the Cancer Genetic Biomarkers for Neuroendocrine Tumor Market. This dominance is fueled by several factors: strong government support for cancer research, a well-established healthcare infrastructure, and a high concentration of leading pharmaceutical and diagnostic companies. However, the Asia Pacific region is expected to experience the fastest growth in this market due to factors like a rising cancer burden, increasing healthcare expenditure, and growing awareness about personalized medicine. Europe also boasts a significant market share due to its advanced healthcare systems and focus on innovation. While South America, the Middle East, and Africa hold potential for future growth, limitations in infrastructure and research funding currently hinder their dominance in this market.
COVID-19 Impact Analysis on the Global Cancer Genetic Biomarkers For Neuroendocrine Tumor Market
The COVID-19 pandemic delivered a mixed bag of impacts on the Global Cancer Genetic Biomarkers for Neuroendocrine Tumor Market. On the one hand, disruptions to clinical trials and delays in non-essential procedures likely slowed the development and implementation of new genetic tests. Additionally, resource constraints in healthcare systems during the peak of the pandemic may have pushed cancer diagnoses, including those of neuroendocrine tumors, down the priority list. This could have resulted in a temporary dip in demand for genetic biomarker tests. However, there are also positive outtakes. The pandemic heightened awareness of the importance of early cancer detection and the value of precision medicine. This could fuel a future surge in demand for advanced diagnostic tools like genetic biomarkers. Furthermore, the rapid advancements in telemedicine and remote monitoring technologies during COVID-19 could open doors for wider accessibility of genetic testing, potentially expanding the market reach. Overall, the long-term impact of COVID-19 on this market remains to be seen. While initial disruptions were likely felt, the increased focus on early detection and personalized medicine could lead to a positive growth trajectory in the coming years.
Latest trends/Developments
The Cancer Genetic Biomarkers for Neuroendocrine Tumor Market is brimming with exciting developments. One key trend is the growing focus on liquid biopsies. These minimally invasive tests analyze tumor-derived DNA circulating in a patient's blood. This approach offers a simpler and potentially more cost-effective way to obtain genetic information compared to traditional tissue biopsies. Researchers are actively developing liquid biopsy tests specifically tailored to neuroendocrine tumors, aiming for earlier detection and easier treatment monitoring. Another trend is the rise of multi-gene panels. These comprehensive tests analyze a broader spectrum of genes simultaneously, providing a more detailed picture of the tumor's genetic landscape. This can be crucial for identifying rare mutations or uncovering complex interactions between multiple genes that contribute to neuroendocrine tumor development. Additionally, advancements in artificial intelligence (AI) are being harnessed to analyze vast datasets of genetic information. AI algorithms can help identify new biomarkers, predict treatment response, and even personalize treatment plans based on a patient's unique genetic profile. As research continues and these trends gain momentum, the Cancer Genetic Biomarkers for Neuroendocrine Tumor Market is poised to revolutionize the way we diagnose and treat these complex cancers.
Key Players:
-
F. Hoffmann-La Roche Ltd.
-
Abbott Laboratories
-
Illumina, Inc.
-
QIAGEN N.V.
-
Thermo Fisher Scientific Inc.
-
Myriad Genetics, Inc.
-
Roche Diagnostics
-
Agilent Technologies Inc.
-
Merck KGaA, Darmstadt, Germany
-
Invitae Corporation
Chapter 1. Cancer Genetic Biomarkers For Neuroendocrine Tumor Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Cancer Genetic Biomarkers For Neuroendocrine Tumor Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Cancer Genetic Biomarkers For Neuroendocrine Tumor Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Cancer Genetic Biomarkers For Neuroendocrine Tumor Market - Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Cancer Genetic Biomarkers For Neuroendocrine Tumor Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Cancer Genetic Biomarkers For Neuroendocrine Tumor Market – By Type
6.1 Introduction/Key Findings
6.2 Mutation biomarkers
6.3 Gene expression biomarkers
6.4 Y-O-Y Growth trend Analysis By Type
6.5 Absolute $ Opportunity Analysis By Type, 2024-2030
Chapter 7. Cancer Genetic Biomarkers For Neuroendocrine Tumor Market – By Application
7.1 Introduction/Key Findings
7.2 Diagnosis
7.3 Prognosis
7.4 Y-O-Y Growth trend Analysis By Application
7.5 Absolute $ Opportunity Analysis By Application, 2024-2030
Chapter 8. Cancer Genetic Biomarkers For Neuroendocrine Tumor Market , By Geography – Market Size, Forecast, Trends & Insights
8.1 North America
8.1.1 By Country
8.1.1.1 U.S.A.
8.1.1.2 Canada
8.1.1.3 Mexico
8.1.2 By Type
8.1.3 By Application
8.1.4 Countries & Segments - Market Attractiveness Analysis
8.2 Europe
8.2.1 By Country
8.2.1.1 U.K
8.2.1.2 Germany
8.2.1.3 France
8.2.1.4 Italy
8.2.1.5 Spain
8.2.1.6 Rest of Europe
8.2.2 By Type
8.2.3 By Application
8.2.4 Countries & Segments - Market Attractiveness Analysis
8.3 Asia Pacific
8.3.1 By Country
8.3.1.1 China
8.3.1.2 Japan
8.3.1.3 South Korea
8.3.1.4 India
8.3.1.5 Australia & New Zealand
8.3.1.6 Rest of Asia-Pacific
8.3.2 By Type
8.3.3 By Application
8.3.4 Countries & Segments - Market Attractiveness Analysis
8.4 South America
8.4.1 By Country
8.4.1.1 Brazil
8.4.1.2 Argentina
8.4.1.3 Colombia
8.4.1.4 Chile
8.4.1.5 Rest of South America
8.4.2 By Type
8.4.3 By Application
8.4.4 Countries & Segments - Market Attractiveness Analysis
8.5 Middle East & Africa
8.5.1 By Country
8.5.1.1 United Arab Emirates (UAE)
8.5.1.2 Saudi Arabia
8.5.1.3 Qatar
8.5.1.4 Israel
8.5.1.5 South Africa
8.5.1.6 Nigeria
8.5.1.7 Kenya
8.5.1.8 Egypt
8.5.1.9 Rest of MEA
8.5.2 By Type
8.5.3 By Application
8.5.4 Countries & Segments - Market Attractiveness Analysis
Chapter 9. Cancer Genetic Biomarkers For Neuroendocrine Tumor Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
9.1 F. Hoffmann-La Roche Ltd.
9.2 Abbott Laboratories
9.3 Illumina, Inc.
9.4 QIAGEN N.V.
9.5 Thermo Fisher Scientific Inc.
9.6 Myriad Genetics, Inc.
9.7 Roche Diagnostics
9.8 Agilent Technologies Inc.
9.9 Merck KGaA, Darmstadt, Germany
9.10 Invitae Corporation
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The Global Cancer Genetic Biomarkers For Neuroendocrine Tumor Market was valued at USD 15.68 billion in 2023 and will grow at a CAGR of 14.60% from 2024 to 2030. The market is expected to reach USD 40.70 billion by 2030.
Rising Burden of Cancer, Increased Focus on Personalized Medicine, Improved Diagnosis and Early Detection these are the reasons which is driving the market.
Based on Application it is divided into two segments – Diagnosis, Prognosis.
North America is the most dominant region for the luxury vehicle Market.
F. Hoffmann-La Roche Ltd., Abbott Laboratories, Illumina, Inc., QIAGEN N.V., Thermo Fisher Scientific Inc.