Global Biological Safety Testing for Monoclonal Antibodies Market Size (2024-2030)
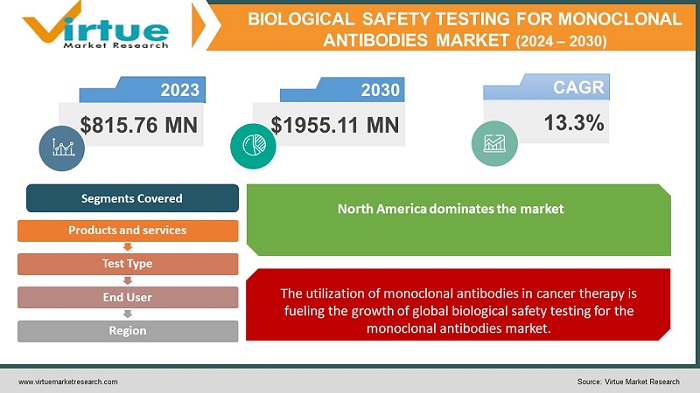
The Global Biological Safety Testing for Monoclonal Antibodies Market was valued at USD 815.76 million and is projected to reach a market size of USD 1955.11 million by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 13.3%.
Monoclonal antibodies are proteins made artificially to look like the antibodies our immune system makes. They work by targeting specific foreign substances called antigens and assisting in their elimination to enhance our immune response. Monoclonal antibodies, in contrast to polyclonal antibodies, which may bind to several antigens and originate from different immune cells, are perfect duplicates of a single antibody that attaches to a single antigen. They can be utilized for a multitude of applications, including laboratory testing, tissue and blood matching for transplants, disease diagnosis, and treatment of various health conditions, such as cancer, organ transplant rejection, inflammatory disorders, infections like COVID-19, osteoporosis, issues with vision, migraines, high cholesterol, and nervous system disorders. Over time, the utilization of monoclonal antibodies in medical therapy has raised gradually.
Monoclonal antibodies are typically administered through intravenous infusion, often in infusion centers alongside other patients. They may be given subcutaneously in certain instances, with patients instructed to self-administer the injections. Monoclonal antibodies can be utilized alone as therapy, combined with other drugs, or engineered to target multiple antigens simultaneously. They offer advantages such as greater precision in treatment, higher effectiveness, and standardized quality across production batches. The ability to produce monoclonal antibodies in large quantities has further expanded their potential applications.
Global Biological Safety Testing for Monoclonal Antibodies Market Drivers:
The utilization of monoclonal antibodies in cancer therapy is fueling the growth of global biological safety testing for the monoclonal antibodies market.
Monoclonal antibodies, lab-made proteins, mimic our natural antibodies and help eliminate disease-causing germs. Like our body's antibodies, they target specific substances. In cancer treatment, monoclonal antibodies are used for targeted therapy. They interact with specific targets to combat cancer cells. Some also serve as immunotherapy, activating the immune system against cancer. For instance, rituximab marks CD20 protein on B cells and certain cancer cells, prompting their elimination. Other antibodies, like blinatumomab, bring T cells close to cancer cells by binding to CD19 and CD3 proteins. This enables T cells to effectively respond and eliminate leukemia cells. Monoclonal antibodies offer promising approaches to cancer treatment by leveraging the immune system's capabilities. Consequently, the demand for biological safety testing for these antibodies is driven by their therapeutic potential.
The authorization of Sotrovimab for the treatment of mild-to-moderate COVID-19 infections is another factor contributing to the growth of the global biological safety testing for the monoclonal antibodies market.
In May 2021, the FDA granted emergency use authorization for Sotrovimab, an investigational monoclonal antibody therapy, to treat mild-to-moderate COVID-19 in adults and pediatric patients (12 years and older, weighing at least 40 kilograms). This authorization is for individuals who have tested positive for SARS-CoV-2 and are at high risk of severe COVID-19, including hospitalization or death, particularly those who are 65 years and older or have specific medical conditions. Sotrovimab targets the spike protein of the virus, aiming to prevent its attachment and entry into human cells. This authorization contributes to the demand for biological safety testing for monoclonal antibodies.

Global Biological Safety Testing for Monoclonal Antibodies Market Challenges:
The global biological safety testing for monoclonal antibodies market is encountering challenges, primarily in terms of the high cost of development and the shortage of skilled professionals. Specific medications like REGEN-COV and Sotrovimab, used for COVID-19 treatment, can be quite expensive, with prices ranging from around USD 1,250 to USD 2,100 per infusion. These elevated prices pose a challenge to accessing mAb therapies, particularly for patients in developing countries, and can limit the widespread adoption of mAbs. Moreover, the biological safety testing process is highly time-consuming and complex, and there aren't enough competent professionals to undertake the testing procedures. Thus, these challenges inhibit the growth of global biological safety testing for the monoclonal antibodies market.
Global Biological Safety Testing for Monoclonal Antibodies Market Opportunities:
Growing CRO outsourcing for biopharmaceutical activities presents a lucrative opportunity in the global biological safety testing for monoclonal antibodies market. Contract research organizations (CROs) serve as external partners for pharmaceutical and biotechnology companies and academic institutions, offering a wide range of services including cell and virus bank characterization, genetic stability testing, analytical services for biologics, and more. Outsourcing to CROs has become a popular choice as advancements in technology make it difficult for companies to handle all testing in-house. CROs can invest in advanced infrastructure and techniques, such as rapid mycoplasma tests and robotic systems, resulting in cost savings and optimal staffing. The outsourcing of safety testing services to CROs provides advantages like reduced costs and commercial sustainability, and as pharmaceutical pipelines expand, the demand for CROs for biologics safety testing is anticipated to grow.
COVID-19 Impact on the Global Biological Safety Testing for Monoclonal Antibodies Market:
The outbreak of the COVID-19 pandemic substantially impacted the global biological safety testing for the monoclonal antibodies market. The pandemic caused disruptions in supply chains and distribution of goods and services, which highly affected the supply of essential materials like reagents and testing equipment for biological safety testing for monoclonal antibodies. This factor negatively impacted the growth of the global biological safety testing for monoclonal antibodies market. However, in response to the urgent need for effective treatments, there has been a rapid development of multiple neutralizing monoclonal antibodies (mAbs) specifically designed to target the SARS-CoV-2 virus. These factors positively impacted the market's growth. Therefore, the global biological safety testing for monoclonal antibodies market experienced both challenges and opportunities during the difficult time of the COVID-19 pandemic.
Global Biological Safety Testing for Monoclonal Antibodies Market Recent Developments:
- In March 2022, Thermo Fisher Scientific and Symphogen, an affiliate of Servier, extended their collaboration to deliver biopharmaceutical labs with cutting-edge tools and streamlined workflows. This partnership aims to enhance the characterization of complex therapeutic proteins, improving efficiency and productivity.
- In July 2021, Lonza Group AG extended its partnership with a major biopharmaceutical company for the large-scale production of monoclonal antibodies. The agreement entails utilizing a marked portion of the capacity at Lonza's Visp facility, which is currently under construction with six 20,000L bioreactors. This collab, along with other customers, now reserves about 75% of the facility's total capacity. The aim is to facilitate efficient and extensive manufacturing of monoclonal antibodies once the product is approved and production is scaled up.
- In July 2020, SGS S.A. signed an agreement with AstraZeneca to supply safety, quality control, and analytical testing services for AZD1222, a COVID-19 vaccine candidate. SGS's Biosafety Centre of Excellence in Glasgow, Scotland, will conduct vaccine bulk harvest and drug substance testing. The expanded laboratory facility is capable of testing cell banks, vaccines, gene and cell therapies, monoclonal antibodies, and other recombinant protein-based biological medicines, including coronavirus vaccines.
BIOLOGICAL SAFETY TESTING FOR MONOCLONAL ANTIBODIES MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
13.3% |
|
Segments Covered |
By Products and services, Test Type, End User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Charles River Laboratories International, Inc. (United States), Lonza Group AG (Switzerland), Thermo Fisher Scientific Inc. (United States), The Merck Group (Germany), SGS S.A. (Switzerland), WuXi AppTec (China), Eurofins Scientific SE (Luxembourg), Bio-Rad Laboratories, Inc. (United States), Nelson Laboratories, LLC (United States), Mabtech AB (Sweden) |
Global Biological Safety Testing for Monoclonal Antibodies Market Segmentation:
Global Biological Safety Testing for Monoclonal Antibodies Market Segmentation: By Products and Services
- Consumables
- Instruments
- Services
In 2022, the consumables segment held the highest market share. The growth can be attributed to the extensive utilization of consumables, such as assays, kits, and reagents, for the detection of various contaminants in test samples. Assays, kits, and reagents are vital components of biological safety testing, serving purposes such as process validation, quality control, raw material testing, and final product release. Biopharmaceutical companies favor kits owing to their convenience, long shelf life, and premeasured components. The reagents market is growing as biosciences and biotechnology finds more applications in the pharmaceutical and healthcare sectors. This expansion is fueled by the increasing demand for reagents in research and testing across various activities.
Global Biological Safety Testing for Monoclonal Antibodies Market Segmentation: By Test Type
- Bioburden Tests
- Endotoxin Tests
- Sterility Tests
- Others
In 2022, the bioburden tests segment held the highest market share. The growth can be attributed to the increasing demand for proper sterilization, guided by organizations like the World Health Organization (WHO. Contamination can come from various sources, leading to variations in bioburden levels between product batches. Routine testing is essential for quality control, detecting and quantifying microbial contamination throughout production. Bioburden testing is a vital quality control procedure that assesses microbial contamination at different production stages. Accurate testing and quality control are crucial to minimizing consumer risks, particularly in regulated environments.
Global Biological Safety Testing for Monoclonal Antibodies Market Segmentation: By End-User
- Academic and Research Institutes
- Contract Development and Manufacturing Companies
- Biotechnology and Pharmaceutical Companies
Based on the end-user, the global biological safety testing for monoclonal antibodies market is segmented into academic and research institutes, contract development and manufacturing companies, and biotechnology and pharmaceutical companies. In 2022, the pharmaceutical and biotechnology companies segment held the highest market share. The growth can be attributed to their large involvement in development and production, adherence to regulatory requirements, and in-house testing capabilities. These businesses invest resources in research, manufacturing, and commercialization, requiring comprehensive safety testing to ensure quality and efficacy. Strict regulations from authorities like the Food and Drug Administration (FDA) and European Medicines Agency (EMA) necessitate robust testing for regulatory compliance. In-house laboratories allow for greater control and potential cost reduction.
Global Biological Safety Testing for Monoclonal Antibodies Market Segmentation: By Region
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
The region of North America held the largest share of the global biological safety testing for monoclonal antibodies market in the year 2022. The rising incidence of health conditions, such as cancer, organ transplant rejection, inflammatory disorders, COVID-19 infection, osteoporosis, eye issues, migraines, high cholesterol, and nervous system disorders, the presence of well-established healthcare infrastructure in nations, such as the United States and Canada, and the increasing investments in research and development activities are some of the factors propelling the region's growth. Additionally, North America is home to several significant market players, including Charles River Laboratories International, Inc., Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., Nelson Laboratories, LLC, and Laboratory Corporation.
Due to the growing biotechnology and pharmaceutical industries in nations, such as Germany, the United Kingdom, and France, the existence of supportive government policies and initiatives, and the strong presence of major market players, including Lonza Group AG, The Merck Group, and SGS S.A., the region of Europe is anticipated to expand at the quickest rate over the forecast period.
Global Biological Safety Testing for Monoclonal Antibodies Market Key Players:
- Charles River Laboratories International, Inc. (United States)
- Lonza Group AG (Switzerland)
- Thermo Fisher Scientific Inc. (United States)
- The Merck Group (Germany)
- SGS S.A. (Switzerland)
- WuXi AppTec (China)
- Eurofins Scientific SE (Luxembourg)
- Bio-Rad Laboratories, Inc. (United States)
- Nelson Laboratories, LLC (United States)
- Mabtech AB (Sweden)
Chapter 1. BIOLOGICAL SAFETY TESTING FOR MONOCLONAL ANTIBODIES MARKET – Scope & Methodology
1.1. Market Segmentation
1.2. Assumptions
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2. BIOLOGICAL SAFETY TESTING FOR MONOCLONAL ANTIBODIES MARKET – Executive Summary
2.1. Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-19 Impact Analysis
2.3.1. Impact during 2024 – 2030
2.3.2. Impact on Supply – Demand
Chapter 3. BIOLOGICAL SAFETY TESTING FOR MONOCLONAL ANTIBODIES MARKET – Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4. BIOLOGICAL SAFETY TESTING FOR MONOCLONAL ANTIBODIES MARKET - Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatory Scenario - By Region
4.3 Customer Analysis
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. BIOLOGICAL SAFETY TESTING FOR MONOCLONAL ANTIBODIES MARKET - Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6. BIOLOGICAL SAFETY TESTING FOR MONOCLONAL ANTIBODIES MARKET – by Products and Services
6.1. Consumables
6.2. Instruments
6.3. Services
Chapter 7. BIOLOGICAL SAFETY TESTING FOR MONOCLONAL ANTIBODIES MARKET – By Test Type
7.1. Bioburden Tests
7.2. Endotoxin Tests
7.3. Sterility Tests
7.4. Others
Chapter 8. BIOLOGICAL SAFETY TESTING FOR MONOCLONAL ANTIBODIES MARKET – By End-user
8.1. Academic and Research Institutes
8.2. Contract Development and Manufacturing Companies
8.3. Biotechnology and Pharmaceutical Companies
Chapter 9. BIOLOGICAL SAFETY TESTING FOR MONOCLONAL ANTIBODIES MARKET – By Region
9.1. North America
9.2. Europe
9.3.The Asia Pacific
9.4.Latin America
9.5. Middle-East and Africa
Chapter 10. BIOLOGICAL SAFETY TESTING FOR MONOCLONAL ANTIBODIES MARKET– Company Profiles – (Overview, Product Portfolio, Financials, Developments)
10.1. Charles River Laboratories International, Inc. (United States)
10.2. Lonza Group AG (Switzerland)
10.3. Thermo Fisher Scientific Inc. (United States)
10.4. The Merck Group (Germany)
10.5. SGS S.A. (Switzerland)
10.6. WuXi AppTec (China)
10.7. Eurofins Scientific SE (Luxembourg)
10.8. Bio-Rad Laboratories, Inc. (United States)
10.9. Nelson Laboratories, LLC (United States)
10.10. Mabtech AB (Sweden)
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The Global Biological Safety Testing for Monoclonal Antibodies Market was valued at USD 815.76 million and is projected to reach a market size of USD 1955.11 million by the end of 2030. Over the forecast period of 2024-2030, the market is projected to grow at a CAGR of 13.3%.
The Global Biological Safety Testing for Monoclonal Antibodies Market Drivers are the Utilization of Monoclonal Antibodies in Cancer Therapy and the Authorization of Sotrovimab for the Treatment of Mild-to-Moderate COVID-19 Infections
Based on the Products and Services, the Global Biological Safety Testing for Monoclonal Antibodies Market is segmented into Consumables, Instruments, and Services.
The United States is the most dominating country in the region of North America for the Global Biological Safety Testing for Monoclonal Antibodies Market.
Charles River Laboratories International, Inc., Lonza Group AG, and Thermo Fisher Scientific Inc. are the leading players in the Global Biological Safety Testing for Monoclonal Antibodies Market.



