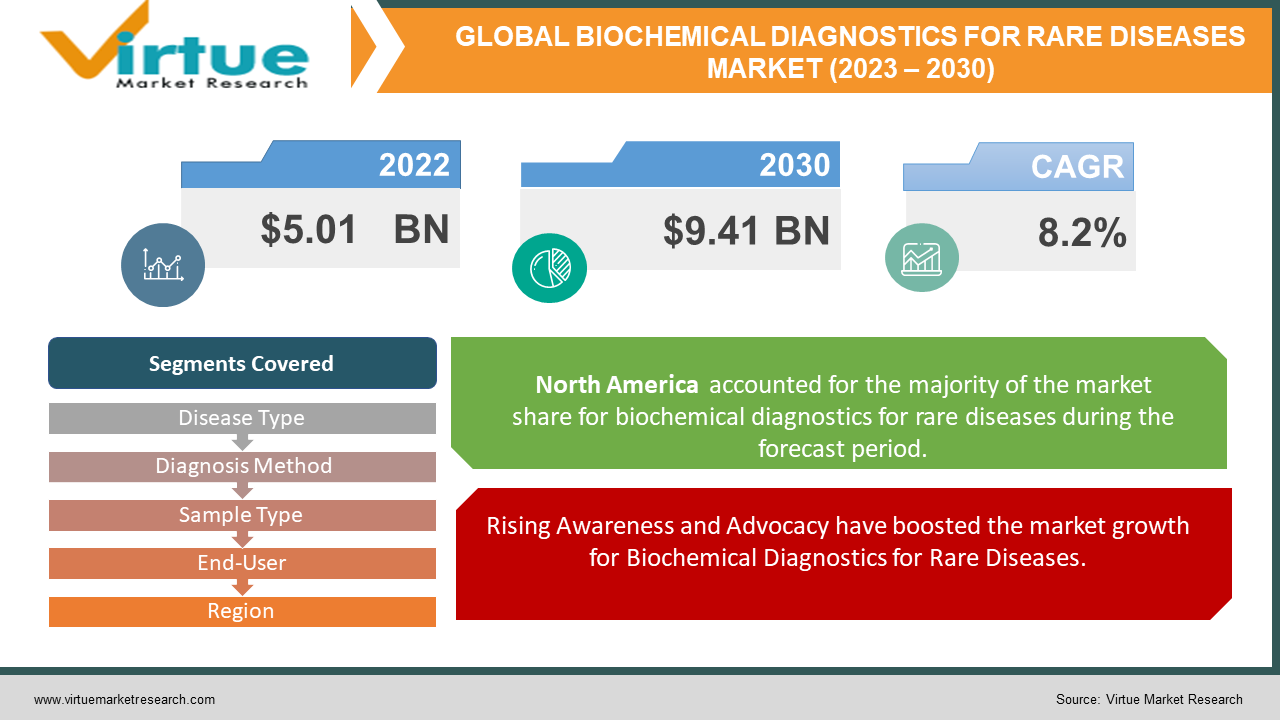
Biochemical Diagnostics for Rare Diseases Market Size (2023-2030)
The Global Biochemical Diagnostics for Rare Diseases Market was valued at USD 5.01 billion in 2022 and is projected to reach USD 9.41 billion by 2030, exhibiting a robust CAGR of 8.2% during the forecast period 2023-2030.
Biochemical diagnostics for rare diseases encompass advanced testing techniques that aim to identify and diagnose uncommon disorders, often with a genetic basis. These tests meticulously analyze distinct biomarkers, metabolites, and genetic markers associated with these conditions, thereby facilitating precise and early detection. This field is pivotal in addressing the pressing demand for accurate diagnostics and individualized treatment strategies for individuals grappling with rare diseases. By meticulously scrutinizing unique biomarkers, healthcare professionals can discern deviations from the norm, aiding in the early recognition of potential rare disorders. The scrutiny of metabolites provides a window into an individual's physiological landscape, uncovering anomalies that could serve as indicators of an underlying rare condition. Equally vital are genetic markers, as cutting-edge genetic testing methods can unearth mutations or variations that are indicative of particular rare diseases. This holistic approach of biochemical diagnostics caters to personalized care by enabling prompt identification and targeted therapeutic interventions. Consequently, this approach not only elevates the quality of life for those affected by rare diseases but also holds the promise of enhanced outcomes as technological advancements and research continue to propel the field forward.
Global Biochemical Diagnostics for Rare Diseases Market Drivers:
Rising Awareness and Advocacy have boosted the market growth for Biochemical Diagnostics for Rare Diseases.
The growing emphasis on raising awareness about rare diseases within the healthcare community, among patients, and regulatory authorities has ignited a surge in the requirement for tailored diagnostics. This surge is greatly attributed to the collective endeavors of advocacy organizations and governmental bodies, aimed at bridging the diagnostic voids prevalent in the realm of rare diseases, thereby driving the expansion of the market.
Advancements in Technology have enhanced the efficiency of Biochemical Diagnostics for Rare Diseases.
Advancements in molecular diagnostics, genetic sequencing, and metabolomics have revolutionized rare disease identification. Innovative techniques like Next-generation sequencing (NGS), mass spectrometry, and advanced imaging have significantly improved the accuracy and efficiency of biochemical diagnostics for rare diseases. These technologies enable earlier and more precise detection, leading to better outcomes for individuals affected by these conditions.
The personalized Medicine Paradigm has fuelled the demand for comprehensive diagnostic approaches.
The rise of personalized medicine has propelled the use of biochemical diagnostics in rare diseases. Individualized treatment strategies demand accurate disease profiling, driving the need for comprehensive diagnostic approaches. This trend recognizes the uniqueness of each patient's condition and requires advanced biochemical testing techniques such as genetic sequencing, biomarker analysis, and metabolomics. These approaches enhance diagnosis precision, enabling tailored and effective treatment plans, thus improving outcomes for individuals affected by rare diseases.
Global Biochemical Diagnostics for Rare Diseases Market Challenges:
Limited Disease Understanding hampers the development of diagnostic assays for Rare Diseases.
The growth of the biochemical diagnostics market for rare diseases is hindered by a limited understanding of these conditions. The intricate and diverse nature of rare diseases poses challenges in deciphering their underlying biochemical mechanisms. This complexity, in turn, obstructs the development of specialized diagnostic assays tailored to these unique disorders, impacting the advancement of this market segment.
High Cost for developing Biochemical Diagnostic tests hinders inclusivity for the final product.
The progress of the biochemical diagnostics market for rare diseases is impeded by the considerable expenses associated with test development. Creating and bringing to market advanced diagnostic tests demands significant investments in research, technology, and clinical validation. These elevated costs can limit accessibility to the final product, particularly in regions with limited resources. This financial barrier could curtail the broader adoption of these vital diagnostic tools within the rare diseases market.
The lack of standardized diagnostics poses critical challenges to regulatory approval for Biochemical Diagnostics for Rare Diseases.
Navigating the biochemical diagnostics market for rare diseases is complicated by the absence of uniform diagnostic criteria. The unique nature of these diseases presents hurdles in establishing consistent criteria, which in turn creates challenges during the regulatory approval process. Meeting stringent regulatory standards necessitates not only accurate and valid testing but also aligning with varying and evolving criteria. This underscores the complexity of ensuring reliable and effective diagnostic solutions for rare diseases, highlighting the need for both scientific innovation and regulatory cooperation to overcome these challenges.
Global Biochemical Diagnostics for Rare Diseases Market Opportunities:
Collaborative Research Initiatives facilitate new diagnostic methods for Rare Diseases.
Collaborative research initiatives play a pivotal role in advancing diagnostic methods for rare diseases. By fostering partnerships among research institutions, diagnostic companies, and healthcare providers, these initiatives accelerate the exploration of novel biomarkers and diagnostic techniques. The complex nature of rare diseases demands a multidisciplinary approach, where diverse expertise converges to develop innovative solutions. Through collective efforts, these collaborations not only drive the discovery of accurate and efficient diagnostic tools but also contribute to the growth of the biochemical diagnostics market for rare diseases.
Technological Innovations offer opportunities for developing novel Biochemical Diagnostic tools for Rare Diseases.
Technological innovations are opening avenues for creating groundbreaking biochemical diagnostic tools for rare diseases. The relentless progress in high-throughput sequencing, metabolomics, and data analytics provides a fertile ground for the emergence of novel diagnostic approaches. These innovations empower researchers and clinicians to delve deeper into the intricate biochemical intricacies of rare diseases, potentially unveiling new biomarkers and diagnostic methods. By harnessing these advancements, the biochemical diagnostics market for rare diseases stands to witness transformative growth.
Personalized Treatment Adoption creates a favorable environment for the growth of biochemical diagnostics for rare diseases.
The expansion of personalized treatment strategies and the growing focus on early intervention is fostering a conducive environment for the biochemical diagnostics market in the realm of rare diseases. With healthcare shifting towards tailored therapies, the demand for precise and early diagnostic methods is escalating. Biochemical diagnostics play a critical role in enabling targeted interventions by providing accurate disease characterization. This aligns with the paradigm of personalized medicine and positions the market for biochemical diagnostics to thrive and contribute to the advancement of rare disease management.
BIOCHEMICAL DIAGNOSTICS FOR RARE DISEASES MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2022 - 2030 |
|
Base Year |
2022 |
|
Forecast Period |
2023 - 2030 |
|
CAGR |
8.2% |
|
By Disease Type, Diagnosis Method, Sample Type, End-User, and Region |
|
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Thermo Fisher Scientific, HORIBA, Xylem Analytics, Kova International, ARCHIMEDlife |
Biochemical Diagnostics for Rare Diseases Market Segmentation – By Disease Type
-
Metabolic Disorders
-
Lysosomal Storage Disorders
-
Muscle Disorders
-
Immunodeficiencies
-
Leukodystrophies/Genetic Leukoencephalopathies
-
Hemoglobinopathies
-
Pharmacogenetics
-
Others
Based on market segmentation by Disease type, metabolic disorders drive the largest market share for Biochemical diagnostics for Rare Diseases. Metabolic disorders encompass a wide range of conditions that affect the body's ability to process various substances, such as carbohydrates, amino acids, and fats. These disorders can result in a variety of symptoms and health issues. However, Leukodystrophies/Genetic Leukoencephalopathies is the fastest growing segment, moreover due to advancements in genetic testing technologies and the increasing importance of personalized medicine the growth opportunities for Biochemical Diagnostics in Leukodystrophies provide a promising future.
Biochemical Diagnostics for Rare Diseases Market Segmentation – By Diagnosis Method
-
Mass spectrometry
-
Genetic testing
-
PCR
-
Next-generation sequencing (NGS)
-
Microarrays
-
-
Enzyme assays
-
Immunoassays
-
Liquid chromatography
-
Spectrophotometry
-
Others
Based on market segmentation by Diagnosis Method, Genetic Testing methods have been instrumental in capturing a significant market share within the biochemical diagnostics for rare diseases market. The growth can be attributed to the sub-segments – NGS and PCR. These methods are widely used for identifying genetic mutations, variations, and markers associated with rare diseases, enabling accurate and early diagnosis. However, Mass spectrometry has seen the fastest growth in diagnosing rare diseases. It allows for the identification and quantification of molecules based on their mass and charge. Mass spectrometry is particularly valuable in analyzing metabolites and biomarkers, aiding in the diagnosis of metabolic and lysosomal storage disorders.
Biochemical Diagnostics for Rare Diseases Market Segmentation – By Sample Type
-
Dried Blood Spots (DBS)
-
Buccal Swab
-
Urine
-
Cerebrospinal Fluid (CSF)
-
Tissue Biopsy
-
Others
Based on market segmentation by Sample type, Dried Blood Spots have the largest market in biochemical diagnostics for rare diseases due to their numerous advantages like minimal invasiveness, stability, and versatility. DBS simplifies sample collection, especially for infants and children. Its room temperature stability aids transportation and storage, supporting resource-constrained regions. Widely used in newborn screening and research, DBS's significance has propelled its market share, albeit subject to evolving trends and innovations.
Biochemical Diagnostics for Rare Diseases Market Segmentation – By End-User
-
Hospitals
-
Diagnostic Laboratories
-
Research Institutions
-
Others
Based on segmentation by End-user, Hospitals have the largest share in the biochemical diagnostics for rare diseases market. Hospitals serve as primary points of patient care, where diagnosis and treatment take place. Within the intricate landscape of rare diseases, hospitals play an indispensable role in ensuring accurate and timely diagnoses and providing patients with specialized care and personalized treatment plans. However, the Diagnostic laboratories category is the fastest growing. Whether independent or affiliated with hospitals, Diagnostic Laboratories are equipped with advanced equipment and specialized expertise to conduct a wide range of diagnostic tests. They often handle samples collected from various sources, including blood, urine, tissues, and more. These laboratories use state-of-the-art technologies, such as genetic testing methods, mass spectrometry, and immunoassays, to identify specific biomarkers, mutations, and variations associated with rare diseases.
Biochemical Diagnostics for Rare Diseases Market Segmentation – By Region
-
North America
-
Europe
-
Asia-Pacific
-
Middle South America
-
East and Africa
Based on segmentation by Region, North America has the largest market share owing to its robust healthcare systems, extensive research institutions, and significant investments in healthcare technologies. Moreover, the integration of state-of-the-art diagnostic technologies and a robust network of healthcare facilities has paved the way for accurate and timely diagnoses of rare diseases. Advanced genetic testing methods, mass spectrometry, and high-throughput sequencing technologies are widely employed, allowing for the precise identification of genetic mutations and biomarkers associated with rare conditions.
However, The Asia-Pacific region has shown the fastest growth in biochemical diagnostics for rare diseases market owing to increasing healthcare investments, growing awareness about rare diseases, collaborative research efforts, regulatory advancements, and the region's diverse population that is driving the expansion of the market.
Recent Industry Developments:
In July 2023, The UK Rare Disease Research Platform was established with a £14 million investment over five years by the Medical Research Council (MRC) and the National Institute for Health and Care Research (NIHR). The platform aims to bring together expertise from across the UK’s rare disease research system to foster new and innovative treatments for those directly and indirectly impacted by rare conditions.
Biochemical Diagnostics for Rare Diseases Market Segmentation – By Key Market Players
-
Thermo Fisher Scientific
-
HORIBA
-
Xylem Analytics
-
Kova International
-
ARCHIMEDlife
Chapter 1. Biochemical Diagnostics for Rare Diseases Market – Scope & Methodology
1.1 Market Segmentation
1.2 Assumptions
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Biochemical Diagnostics for Rare Diseases Market – Executive Summary
2.1 Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.3 COVID-19 Impact Analysis
2.3.1 Impact during 2023 – 2030
2.3.2 Impact on Supply – Demand
Chapter 3. Biochemical Diagnostics for Rare Diseases Market – Competition Scenario
3.1 Market Share Analysis
3.2 Product Benchmarking
3.3 Competitive Strategy & Development Scenario
3.4 Competitive Pricing Analysis
3.5 Supplier - Distributor Analysis
Chapter 4. Biochemical Diagnostics for Rare Diseases Market - Entry Scenario
4.1 Case Studies – Start-up/Thriving Companies
4.2 Regulatory Scenario - By Region
4.3 Customer Analysis
4.4 Porter's Five Force Model
4.4.1 Bargaining Power of Suppliers
4.4.2 Bargaining Powers of Customers
4.4.3 Threat of New Entrants
4.4.4 .Rivalry among Existing Players
4.4.5 Threat of Substitutes
Chapter 5. Biochemical Diagnostics for Rare Diseases Market - Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Biochemical Diagnostics for Rare Diseases Market - By Disease Type
6.1 Metabolic Disorders
6.2 Lysosomal Storage Disorders
6.3 Muscle Disorders
6.4 Immunodeficiencies
6.5 Leukodystrophies/Genetic Leukoencephalopathies
6.6 Hemoglobinopathies
6.7 Pharmacogenetics
6.8 Others
Chapter 7. Biochemical Diagnostics for Rare Diseases Market - By Diagnosis Method
7.1 Mass spectrometry
7.2 Genetic testing
7.2.1 PCR
7.2.2 Next-generation sequencing (NGS)
7.2.3 Microarrays
7.3 Enzyme assays
7.4 Immunoassays
7.5 Liquid chromatography
7.6 Spectrophotometry
7.7 Others
Chapter 8. Biochemical Diagnostics for Rare Diseases Market - By Sample Type
8.1 Dried Blood Spots (DBS)
8.2 Buccal Swab
8.3 Urine
8.4 Cerebrospinal Fluid (CSF)
8.5 Tissue Biopsy
8.6 Others
Chapter 9. Biochemical Diagnostics for Rare Diseases Market - By End-User
9.1 Hospitals
9.2 Diagnostic Laboratories
9.3 Research Institutions
9.4 Others
Chapter 10. Biochemical Diagnostics for Rare Diseases Market – By Region
10.1 North America
10.2 Europe
10.3 Asia-Pacific
10.4 Latin America
10.5 The Middle East
10.6 Africa
Chapter 11. Biochemical Diagnostics for Rare Diseases Market – Key Players
11.1 Thermo Fisher Scientific
11.2 HORIBA
11.3 Xylem Analytics
11.4 Kova International
11.5 ARCHIMEDlife
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The global Biochemical Diagnostics for Rare Diseases Market was valued at USD 5.01 billion in 2022 and is projected to reach USD 9.41 billion by 2030, exhibiting a robust CAGR of 8.2% during the forecast period 2023-2030.
The drivers include rising awareness about rare diseases, technological advancements, and the personalized medicine paradigm.
North America dominates the market in Global Biochemical Diagnostics for Rare Diseases owing to its robust healthcare systems, extensive research institutions, and significant investments in healthcare technologies.