Big Data in Vaccine Development Market Size (2024 – 2030)
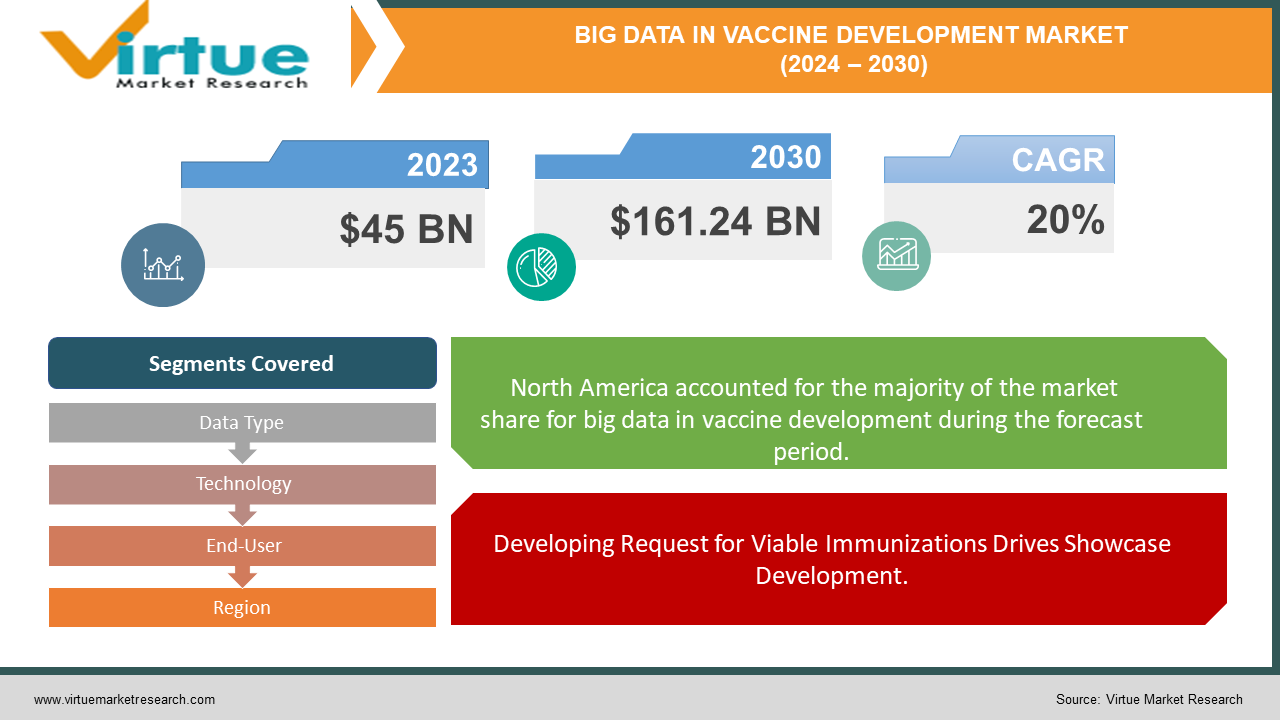
The market for big data in the vaccine development market was estimated to be worth USD 45 billion in 2023 and is expected to increase to USD 161.24 billion by 2030, with a projected compound annual growth rate (CAGR) of 20% from 2024 to 2030.
The advertisement for Huge Information in Antibody Advancement is at the bleeding edge of advancement, leveraging progressed information analytics methods to revolutionize antibody advancement preparation. With the merging of enormous information analytics, bioinformatics, and biotechnology, this advertisement is encountering quick development and change. Huge information arrangements are progressively utilized over different stages of immunization improvement, counting antibody plans, clinical trial optimization, fabricating handle enhancement, and post-market observation. The showcase is driven by variables such as the developing request for viable antibodies against developing irresistible infections, progressions in information science and fake insights, and expanding collaborations between pharmaceutical companies, inquiries about teaching, and innovation suppliers.
Key Insights:
Big data analytics are being increasingly adopted across all phases of vaccine development, with a 30% increase in the utilization of data-driven approaches in clinical trial optimization and a 40% increase in real-time monitoring of vaccine safety and efficacy during post-market surveillance. Collaborative initiatives between pharmaceutical companies, research institutions, and technology providers have flourished, with over 100 strategic partnerships established in 2022 alone, fostering innovation, knowledge exchange, and resource sharing in the market. Despite the advancements, data security concerns remain a challenge, with 45% of industry stakeholders expressing apprehensions regarding data breaches and privacy risks. Implementing robust encryption protocols, stringent access controls, and compliance with data protection regulations can mitigate these concerns and enhance market confidence in data-driven vaccine development initiatives.
Global Big Data in Vaccine Development Market Drivers:
Developing Request for Viable Immunizations Drives Showcase Development.
One of the essential drivers of the worldwide Enormous Information in Immunization Advancement advertise is the expanding request for compelling immunizations to combat rising irresistible infections and open well-being challenges. With the continuous COVID-19 underscoring the basic significance of antibody advancement, there's increased worldwide consideration on leveraging progressed information analytics and huge information advances to speed up the revelation, advancement, and dissemination of immunizations. This request is driving speculations in investigation and advancement, collaborative associations, and imaginative data-driven approaches over the immunization advancement environment, moving to advertise extension.
Quickened Mechanical Progressions Upgrade Immunization Revelation.
Innovative headways in enormous information analytics, counterfeit insights, and machine learning are playing an essential part in driving advancement and effectiveness in immunization improvement forms. By tackling the control of huge information, analysts can analyze tremendous sums of genomic, clinical, and epidemiological information to distinguish potential antibody candidates, foresee resistant reactions, and optimize clinical trial plans, and screen immunization security and adequacy in real time. These progressions empower analysts to speed up immunization revelation timelines, upgrade the exactness of antibody advancement endeavors, and address complex open well-being challenges more viably, driving advertise development and selection of huge information arrangements in immunization advancement.
Collaborative Activities Cultivate Advancement and Information Trade.
Collaborative activities between pharmaceutical companies, investigative education, government offices, and innovation suppliers are cultivating development, information trade, and asset sharing within the Enormous Information in Antibody Advancement market. By pooling ability, information assets, and foundation, partners can quicken antibody disclosure, optimize clinical trial operations, and progress antibody conveyance procedures. These collaborative endeavors empower analysts to use differing datasets, get to cutting-edge advances, and overcome asset imperatives, eventually driving headways in immunization improvement and reinforcing the worldwide open well-being reaction to irresistible maladies.
Global Big Data in Vaccine Development Market Restraints and Challenges:
Information Security and Security Concerns Block Advance.
One of the noteworthy challenges confronting the worldwide Enormous Information in Immunization Improvement showcase is the developing concern over information security and security. As the volume and complexity of information utilized in antibody improvement increment, partners confront challenges in guaranteeing the protection and security of touchy data. Information breaches, unauthorized get to, and abuse of individual well-being data pose noteworthy dangers, undermining belief and certainty in data-driven immunization advancement activities. Tending to these concerns requires strong information assurance measures, counting encryption conventions, getting to controls, and compliance with administrative systems such as GDPR and HIPAA, to protect understanding protection and relieve security dangers successfully.
Integration and Standardization Issues Constrain Information Utility.
Another challenge within the Huge Information in Antibody Advancement showcase is the need for integration and standardization of information sources and groups. The different natures of information sources, including genomic information, clinical trial information, and epidemiological information, postures challenges in information integration, harmonization, and interoperability. Without standardized information groups and interoperable frameworks, analysts confront challenges in getting to and utilizing differing datasets viably, ruining data-driven bits of knowledge and advancement in immunization advancement. Tending to these integration and standardization issues requires collaborative endeavors to set up common information measures, interoperable stages, and information-sharing systems, empowering consistent trade and utilization of information over the immunization advancement biological system.
Asset and Framework Limitations Hinder Openness.
Asset and framework limitations pose critical obstructions to the availability and appropriation of enormous information arrangements in immunization advancement, especially in low-resource settings and creating nations. Restricted access to progressed information analytics instruments, high-performance computing framework, and gifted faculty prevent the execution of huge data-driven approaches in immunization inquiry and improvement. Also, aberrations in advanced foundations and networks encourage compound imbalances in getting to and leveraging enormous information for immunization advancement activities. Tending to these challenges requires ventures in capacity building, innovation exchange, and infrastructure development to engage analysts and healthcare experts with the essential assets and capabilities to saddle the potential of huge information in immunization advancement and guarantee evenhanded get to life-saving antibodies.
Global Big Data in Vaccine Development Market Opportunities:
Quickened Immunization Advancement Timelines through Data-Driven Approaches.
One of the noteworthy openings within the worldwide Enormous Information in Immunization Advancement showcase lies in quickening immunization advancement timelines through data-driven approaches. By leveraging progressed huge information analytics, fake insights, and machine learning calculations, analysts can assist in immunization disclosure, planning, and optimization forms. Prescient modeling, virtual screening, and in silico trials empower analysts to recognize promising antibody candidates, foresee resistant reactions, and optimize antibody details more proficiently. These data-driven approaches have the potential to abbreviate advancement timelines, diminish costs, and address critical open well-being needs, displaying lucrative opportunities for partners within the immunization improvement environment.
Personalized Antibodies Custom Fitted to Individual Immune Reactions.
Another promising opportunity within the Enormous Information in Immunization Advancement showcase is the potential for personalized immunizations custom-made to person-resistant reactions. With the appearance of exactness medication and genomic sequencing advances, analysts can analyze a person's hereditary profiles and resistant marks to create personalized immunization procedures. Enormous information analytics empower the distinguishing proof of hereditary varieties, biomarkers, and safe connects of assurance, permitting for the plan of antibodies that inspire focused on safe reactions and give upgraded viability and security profiles. Personalized immunizations offer the potential to progress antibody viability, decrease unfavorable responses, and address antibody reluctance by catering to a person's hereditary and immunological differences.
Real-Time Reconnaissance and Reaction to Rising Irresistible Illnesses.
The worldwide Enormous Information in Antibody Improvement advertisement presents openings for real-time reconnaissance and reaction to developing irresistible infections. By leveraging enormous information analytics, epidemiological observation frameworks, and computerized well-being innovations, analysts can screen illness flare-ups, track transmission designs, and evaluate antibody viability in real time. Early location of rising dangers empowers convenient open well-being mediations, such as focused on inoculation campaigns, control measures, and observation of antibody breakthrough infections. Real-time data-driven reconnaissance frameworks improve widespread readiness and reaction capabilities, empowering proactive procedures to moderate the spread of infectious illnesses and minimize the effect on worldwide well-being and security.
BIG DATA IN VACCINE DEVELOPMENT MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
20% |
|
Segments Covered |
By Data Type, Technology, End-User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
IBM Corporation, Microsoft Corporation, Google LLC (Alphabet Inc.), Amazon Web Services, Inc. (AWS), SAS Institute Inc., Oracle Corporation, Cloudera, Inc., Palantir Technologies Inc., QIAGEN, Freenome Holdings, Inc., Tempus Labs, Inc., Datavant |
Big Data in Vaccine Development Market Segmentation: By Data Type
-
Genomic data
-
Clinical trial data
-
Epidemiological data
-
Manufacturing data
-
Real-world evidence (RWE) data
Within the division of the Enormous Information in Antibody Advancement advertise by information sort, clinical trial information emerges as one of the foremost successful sections due to its basic part in advising immunization improvement forms. Clinical trial information gives priceless experiences into the security, viability, and immunogenicity of antibody candidates through controlled experimentation including human subjects. By analyzing clinical trial information, analysts can assess immunization reactions, evaluate antagonistic responses, and optimize dosing regimens, in this manner progressing the improvement and administrative endorsement of immunizations. Besides, clinical trial information empowers analysts to distinguish subpopulations with shifting resistant reactions, educating focused on antibody methodologies and personalized pharmaceutical approaches. With the expanding accentuation on evidence-based medication and administrative prerequisites for thorough clinical testing, the compelling utilization of clinical trial information is fundamental for quickening antibody improvement timelines and guaranteeing the conveyance of secure and effective antibodies to the worldwide populace.
Big Data in Vaccine Development Market Segmentation: By Technology
-
Big data analytics
-
Artificial intelligence (AI) and machine learning (ML)
-
Data Integration and management systems
-
Predictive modeling tools
-
Data visualization platforms
Artificial Intelligence (AI) and machine learning (ML) stand out as one of the foremost compelling sections and are estimated to be the fastest-growing segment during the forecast period, 2024-2030. AI and ML innovations play a significant part in extricating profitable experiences from tremendous and complex datasets, encouraging data-driven decision-making in immunization advancement. These progressed advances empower analysts to analyze different information sources, counting genomic information, clinical trial information, and epidemiological information, to distinguish designs, anticipate safe reactions, and optimize immunization definitions. Furthermore, AI and ML calculations can streamline clinical trial plans, persistent enrollment, unfavorable occasion checking, upgrading proficiency, and diminishing costs all through the immunization advancement lifecycle. By leveraging AI and ML capabilities, partners can quicken antibody disclosure timelines, make strides in immunization adequacy and security profiles, and address complex open health challenges more successfully, making them vital instruments within the journey for inventive and life-saving antibodies.
Big Data in Vaccine Development Market Segmentation: By End-User
-
Pharmaceutical and biotechnology companies
-
Research Institutions
-
Academic centers
-
Government agencies
-
Healthcare organizations
Pharmaceutical and biotechnology companies emerge as one of the foremost successful sections. These companies play a central part in immunization improvement, leveraging enormous information analytics and progressed innovations to drive development and bring antibodies from revelation to showcase. With significant assets, framework, and skill, pharmaceutical and biotechnology companies can conduct large-scale clinical trials, contribute to inquiries about advancement, and explore complex administrative pathways for antibody endorsement. Also, these companies have the monetary assets to contribute to cutting-edge advances, information analytics stages, and vital associations to quicken antibody revelation and optimize antibody fabricating forms. By collaborating with scholastic centers, inquiring about education, and government organizations, pharmaceutical and biotechnology companies can use differing datasets and mastery to drive breakthroughs in immunization advancement, eventually conveying secure, viable, and open antibodies to address worldwide well-being challenges.
Big Data in Vaccine Development Market Segmentation: Regional Analysis
-
North America
-
Europe
-
Asia-Pacific
-
South America
-
Middle East & Africa
The advertisement shared by the locale within the Huge Information in Antibody Improvement showcase reflects an assorted scene of selection and venture over diverse topographical ranges. North America leads the showcase with a critical share of 35%, driven by a progressed healthcare framework, solid investigative capabilities, and considerable speculations in innovation and advancement. Europe takes after closely behind with a 30% advertise share, upheld by strong administrative systems, scholastic brilliance, and collaborative investigative initiatives in antibody advancement. Within the energetic Asia-Pacific locale, comprising 25% of the showcase share, nations such as China, India, and Singapore are rising as key players, leveraging quick innovative progressions, government backing, and developing healthcare ventures to drive showcase development. South America and the Center East and Africa districts each hold an unassuming 5% advertise share, characterized by beginning but advancing healthcare frameworks and expanding acknowledgment of the significance of huge information analytics in immunization improvement. Generally, these territorial showcase offers emphasize the worldwide importance of enormous information in antibody advancement endeavors and highlight openings for collaboration and advancement over differing geological districts.
COVID-19 Impact Analysis on the Global Big Data in Vaccine Development Market:
The COVID-19 widespread has significantly affected the worldwide Huge Information in Antibody Improvement showcase, reshaping the scene of antibody investigation, advancement, and dissemination. With the pressing have to create successful immunizations against the novel coronavirus, there has been a phenomenal surge in data-driven approaches and collaborations over the antibody improvement biological system. Huge information analytics, counterfeit insights, and machine learning have played essential parts in quickening immunization revelation timelines, optimizing clinical trial plans, and observing antibody security and viability in real time. In addition, the widespread has impelled speculations in computerized well-being foundation, information-sharing activities, and administrative adaptability to speed up antibody improvement forms. Whereas the widespread has underscored the significance of leveraging huge information in reacting to worldwide well-being emergencies, it has moreover highlighted challenges such as information security concerns, information standardization issues, and evenhanded get immunizations. Moving forward, partners within the Huge Information in Immunization Improvement showcase must proceed to improve, collaborate, and address these challenges to upgrade pandemic preparedness, fortify worldwide wellbeing frameworks, and guarantee evenhanded get to to life-saving immunizations within the confront of future wellbeing dangers.
Latest Trends/ Developments:
Within the energetic scene of the worldwide Huge Information in Immunization Advancement showcase, a few striking patterns and improvements are forming the industry's direction. One conspicuous drift is the expanding selection of real-world proof (RWE) information analytics in immunization inquiry and improvement. RWE information, determined from sources such as electronic wellbeing records, wearable gadgets, and social media stages, gives profitable experiences into immunization viability, security, and understanding results in real-world settings. By saddling RWE analytics, analysts can complement conventional clinical trial information with real-world proof, empowering more comprehensive assessments of antibody execution and educating evidence-based decision-making all through the antibody advancement lifecycle. Furthermore, headways in information integration and interoperability arrangements are encouraging consistent conglomeration and investigation of differing datasets, cultivating collaboration, and quickening development in vaccine advancement. In addition, there's a developing accentuation on information straightforwardness, morals, and administration systems to guarantee dependable information utilization and keep up an open belief in data-driven antibody advancement activities. These patterns emphasize the advancing role of enormous information analytics in forming long-term immunization inquiries, empowering more viable and personalized approaches to tend to worldwide well-being challenges.
Key Players:
-
IBM Corporation
-
Microsoft Corporation
-
Google LLC (Alphabet Inc.)
-
Amazon Web Services, Inc. (AWS)
-
SAS Institute Inc.
-
Oracle Corporation
-
Cloudera, Inc.
-
Palantir Technologies Inc.
-
QIAGEN
-
Freenome Holdings, Inc.
-
Tempus Labs, Inc.
-
Datavant
Chapter 1. Big Data in Vaccine Development Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Big Data in Vaccine Development Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Big Data in Vaccine Development Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Big Data in Vaccine Development Market Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Big Data in Vaccine Development Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Big Data in Vaccine Development Market – By Data Type
6.1 Introduction/Key Findings
6.2 Genomic data
6.3 Clinical trial data
6.4 Epidemiological data
6.5 Manufacturing data
6.6 Real-world evidence (RWE) data
6.7 Y-O-Y Growth trend Analysis By Data Type
6.8 Absolute $ Opportunity Analysis By Data Type, 2024-2030
Chapter 7. Big Data in Vaccine Development Market – By Technology
7.1 Introduction/Key Findings
7.2 Big data analytics
7.3 Artificial intelligence (AI) and machine learning (ML)
7.4 Data Integration and management systems
7.5 Predictive modeling tools
7.6 Data visualization platforms
7.7 Y-O-Y Growth trend Analysis By Technology
7.8 Absolute $ Opportunity Analysis By Technology, 2024-2030
Chapter 8. Big Data in Vaccine Development Market – End User
8.1 Introduction/Key Findings
8.2 Pharmaceutical and biotechnology companies
8.3 Research Institutions
8.4 Academic centers
8.5 Government agencies
8.6 Healthcare organizations
8.7 Y-O-Y Growth trend Analysis End User
8.8 Absolute $ Opportunity Analysis End User, 2024-2030
Chapter 9. Big Data in Vaccine Development Market , By Geography – Market Size, Forecast, Trends & Insights
9.1 North America
9.1.1 By Country
9.1.1.1 U.S.A.
9.1.1.2 Canada
9.1.1.3 Mexico
9.1.2 By Data Type
9.1.3 By Technology
9.1.4 End User
9.1.5 Countries & Segments - Market Attractiveness Analysis
9.2 Europe
9.2.1 By Country
9.2.1.1 U.K
9.2.1.2 Germany
9.2.1.3 France
9.2.1.4 Italy
9.2.1.5 Spain
9.2.1.6 Rest of Europe
9.2.2 By Data Type
9.2.3 By Technology
9.2.4 End User
9.2.5 Countries & Segments - Market Attractiveness Analysis
9.3 Asia Pacific
9.3.1 By Country
9.3.1.1 China
9.3.1.2 Japan
9.3.1.3 South Korea
9.3.1.4 India
9.3.1.5 Australia & New Zealand
9.3.1.6 Rest of Asia-Pacific
9.3.2 By Data Type
9.3.3 By Technology
9.3.4 End User
9.3.5 Countries & Segments - Market Attractiveness Analysis
9.4 South America
9.4.1 By Country
9.4.1.1 Brazil
9.4.1.2 Argentina
9.4.1.3 Colombia
9.4.1.4 Chile
9.4.1.5 Rest of South America
9.4.2 By Data Type
9.4.3 By Technology
9.4.4 End User
9.4.5 Countries & Segments - Market Attractiveness Analysis
9.5 Middle East & Africa
9.5.1 By Country
9.5.1.1 United Arab Emirates (UAE)
9.5.1.2 Saudi Arabia
9.5.1.3 Qatar
9.5.1.4 Israel
9.5.1.5 South Africa
9.5.1.6 Nigeria
9.5.1.7 Kenya
9.5.1.8 Egypt
9.5.1.9 Rest of MEA
9.5.2 By Data Type
9.5.3 By Technology
9.5.4 End User
9.5.5 Countries & Segments - Market Attractiveness Analysis
Chapter 10. Big Data in Vaccine Development Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
10.1 IBM Corporation
10.2 Microsoft Corporation
10.3 Google LLC (Alphabet Inc.)
10.4 Amazon Web Services, Inc. (AWS)
10.5 SAS Institute Inc.
10.6 Oracle Corporation
10.7 Cloudera, Inc.
10.8 Palantir Technologies Inc.
10.9 QIAGEN
10.10 Freenome Holdings, Inc.
10.11 Tempus Labs, Inc.
10.12 Datavant
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The market for big data in the vaccine development market was estimated to be worth USD 45 billion in 2023 and is expected to increase to USD 161.24 billion by 2030, with a projected compound annual growth rate (CAGR) of 20% from 2024 to 2030.
The essential drivers of the worldwide Huge Information in Antibody Advancement advertise incorporate the expanding request for viable antibodies, innovative headways in information analytics, and collaborations between partners to quicken immunization investigation and advancement.
The key challenges confronting the worldwide Huge Information in Immunization Advancement showcase incorporate information protection and security concerns, integration and standardization issues, and asset limitations in low-resource settings.
In 2023, North America held the largest share of the global Big Data in the Vaccine development market.
IBM Corporation, Microsoft Corporation, Google LLC (Alphabet Inc.), Amazon Web Services, Inc. (AWS), SAS Institute Inc., Oracle Corporation, Cloudera, Inc., Palantir Technologies Inc., QIAGEN, Freenome Holdings, Inc., Tempus Labs, Inc., Datavant are the main players.



