Autoantibody-Based Diagnostic Biomarkers Market Size (2024 – 2030)
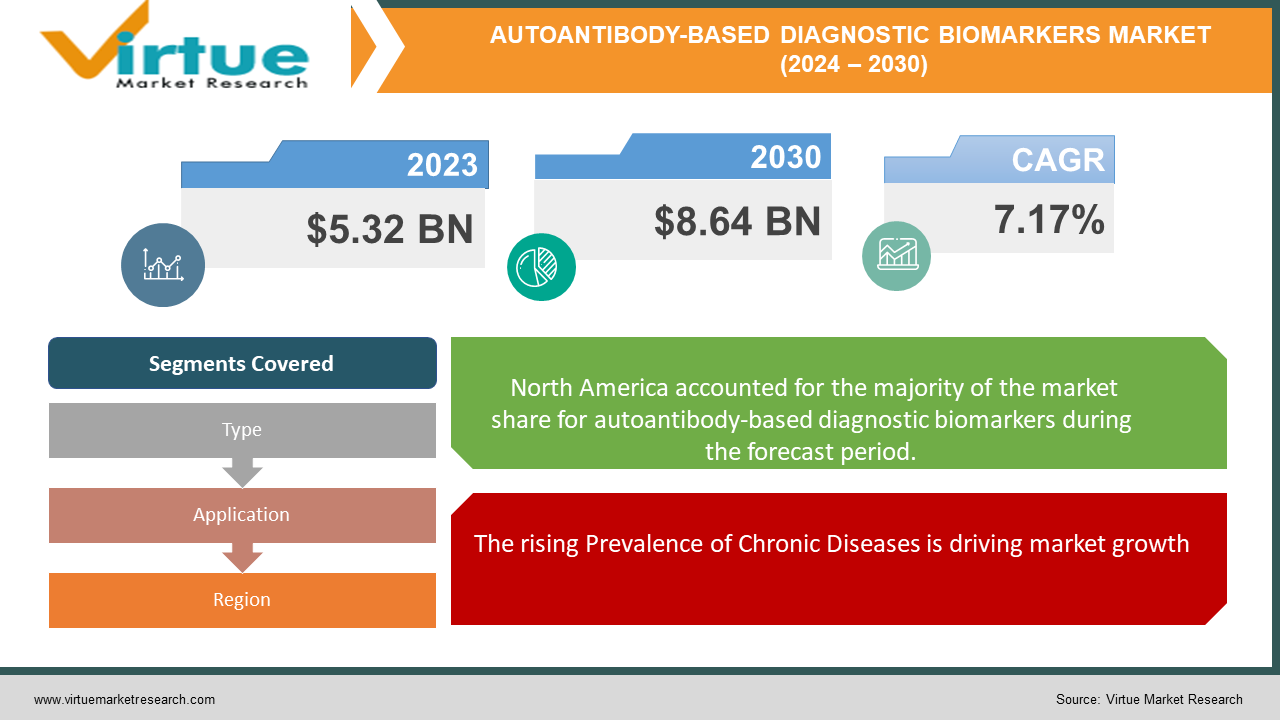
The Global Autoantibody-Based Diagnostic Biomarkers Market was valued at USD 5.32 billion in 2023 and will grow at a CAGR of 7.17% from 2024 to 2030. The market is expected to reach USD 8.64 billion by 2030.
The Autoantibody-Based Diagnostic Biomarkers Market focuses on using antibodies mistakenly produced by the body against itself (autoantibodies) to identify diseases. This rapidly growing field within the biomarker market holds promise for earlier disease detection, allowing for better treatment plans and potentially even predicting future health issues.
Key Market Insights:
Chronic diseases like autoimmune diseases and cancers are becoming increasingly common, affecting millions globally. Autoantibody tests offer the potential for earlier and more accurate detection, which can significantly improve patient outcomes. The rise of personalized medicine emphasizes tailoring treatment plans to individual patients. Autoantibody profiles can provide valuable insights for personalized treatment approaches by identifying the specific immune response at play. Increased public awareness about personalized medicine and the potential of autoantibody testing is driving patient demand for these diagnostics. This can put pressure on healthcare providers to adopt these tests, further propelling market growth.
Global Autoantibody-Based Diagnostic Biomarkers Market Drivers:
The rising Prevalence of Chronic Diseases is driving market growth:
The global rise of chronic diseases like autoimmune conditions and cancers presents a significant challenge for healthcare systems. Aging populations, where the immune system weakens and cellular damage accumulates, are a key contributor. Additionally, modern lifestyle factors like poor diet, lack of exercise, and environmental toxins are believed to play a role. This is where autoantibody tests emerge as a beacon of hope. By detecting antibodies mistakenly produced by the body against its tissues, these tests can identify diseases at an early stage, even before symptoms appear. This early detection window is crucial. It allows for prompt intervention, potentially preventing irreversible damage and significantly improving patient outcomes. For instance, early detection of autoimmune diseases like rheumatoid arthritis can prevent joint destruction, while catching cancers early can lead to more successful treatment options and higher survival rates. Furthermore, autoantibody profiles can provide valuable insights for personalized medicine strategies, allowing doctors to tailor treatment plans to each patient's specific immune response. As awareness of this approach grows among both healthcare professionals and the public, the autoantibody-based diagnostics market is poised for significant expansion, offering a powerful tool to combat the rising tide of chronic diseases.
Improved Diagnostic Accuracy are driving market growth:
Traditional diagnostic methods often rely on a combination of patient symptoms, physical examinations, and broad-spectrum tests. While these methods can be helpful, they may lack precision, leading to misdiagnoses or delayed diagnoses. This is where autoantibody-based biomarkers offer a game-changing advantage. Since autoantibodies are highly specific to certain diseases, their presence can pinpoint the exact issue much earlier and more accurately. Imagine a scenario where a patient experiences fatigue and joint pain. Traditional methods might struggle to differentiate between rheumatoid arthritis, lupus, or even fibromyalgia. However, autoantibody tests can detect the specific antibodies associated with each disease, leading to a swift and precise diagnosis. This early detection window is crucial. By identifying the problem before significant damage occurs, doctors can intervene promptly with targeted treatments. In the case of autoimmune diseases, early intervention can prevent the immune system from attacking healthy tissues, potentially halting or significantly slowing disease progression. Similarly, for cancers, early detection allows for less invasive treatments like surgery or radiation to be more effective. This translates to better patient outcomes, improved quality of life, and potentially reduced healthcare costs associated with managing advanced stages of diseases. As research in this field continues, autoantibody-based biomarkers have the potential to revolutionize diagnostics, empowering doctors to provide faster, more accurate diagnoses for a wider range of conditions.
Increased Focus on Personalized Medicine are driving market growth:
The rise of personalized medicine is revolutionizing healthcare by focusing on individual patients' unique needs. In this paradigm, a one-size-fits-all approach is giving way to treatment plans tailored to a patient's specific genetic makeup and biological response. This is where autoantibody profiles come into play as a powerful tool. By analyzing the specific antibodies a patient produces against their own tissues, doctors gain a unique window into their underlying immune response. Imagine a patient diagnosed with an autoimmune disease. Traditionally, a limited number of treatment options might be available, each with varying degrees of effectiveness and potential side effects. However, an autoantibody profile can reveal which specific molecules are being targeted by the immune system. This information allows doctors to select therapies that specifically target those molecules, potentially leading to more effective and tolerable treatments for the individual patient. Furthermore, autoantibody profiles can help predict a patient's response to treatment. By identifying pre-existing autoantibodies against certain medications, doctors can avoid therapies that might trigger adverse reactions. This personalized approach to treatment selection minimizes trial and error, reduces side effects, and optimizes treatment outcomes for each patient. As research into autoantibodies continues, their role in personalized medicine is expected to grow significantly, paving the way for a future where treatment plans are as unique as the individuals they aim to help.
Global Autoantibody-Based Diagnostic Biomarkers Market challenges and restraints:
High Cost of Development and Testing is a significant hurdle for Autoantibody-Based Diagnostic Biomarkers:
Unlike some traditional diagnostic tests, autoantibody-based biomarkers are intricate. They delve into the body's delicate immune response, requiring sophisticated technologies like protein microarrays and multiplex assays to identify specific autoantibodies. Validating these tests further adds to the cost burden. Clinical trials are essential to ensure accuracy and effectiveness, but they involve recruiting large patient groups, meticulously monitoring results, and often require multiple phases. This translates to significant investments in time and resources. The high costs associated with development and validation act as a barrier to entry, potentially limiting the number of companies and research institutions willing to participate in this field. This can stifle innovation and slow down the discovery of new autoantibody biomarkers for various diseases. However, collaborations between pharmaceutical companies, research institutions, and government agencies can help distribute the financial burden and accelerate advancements in this promising area of diagnostics.
Specificity and Standardization Issues is throwing a curveball at Autoantibody-Based Diagnostic Biomarkers market:
One of the major hurdles in autoantibody-based diagnostics is the inherent variability of the immune response. Unlike a fingerprint, a person's autoantibody profile can be a complex and individualized picture. Even for the same disease, patients might produce different autoantibodies, making it challenging to design tests with sufficient specificity to accurately diagnose a broad range of individuals. This variability necessitates highly specific tests to avoid misdiagnoses. Furthermore, standardizing testing procedures across different laboratories is paramount. Inconsistencies in how samples are prepared, analyzed, and interpreted can lead to discrepancies in results, hindering the test's reliability for widespread clinical use. Researchers are actively developing methods to account for this variability, such as utilizing panels of multiple autoantibodies instead of relying on a single marker. Additionally, efforts are underway to establish standardized testing protocols to ensure consistent and reliable results across various healthcare facilities. Overcoming this variability challenge is crucial to unlocking the full potential of autoantibody-based diagnostics and transforming them into a cornerstone of precision medicine.
Regulatory Hurdles are a growing nightmare for Autoantibody-Based Diagnostic Biomarkers:
A significant hurdle for autoantibody-based diagnostics lies in the regulatory gauntlet new diagnostic tests must navigate. These approval processes, overseen by agencies like the US Food and Drug Administration (FDA), are designed to ensure the safety and efficacy of tests before they reach patients. However, the length and complexity of these processes can be a major bottleneck. Meticulous data collection on test accuracy, safety, and clinical utility through rigorous clinical trials is essential. This can take years to complete and requires significant investment. Regulatory agencies then meticulously evaluate this data, potentially requesting additional studies or modifications before granting approval. While these measures are vital for patient safety, the lengthy timelines can significantly delay the market entry of promising autoantibody-based biomarkers. This delay can stifle innovation and limit patient access to potentially life-saving diagnostic tools. Streamlining the regulatory process, potentially through dedicated pathways for novel diagnostics, could be an avenue to expedite approval times and accelerate the impact of autoantibody-based tests in clinical practice.
Market Opportunities:
The Autoantibody-Based Diagnostic Biomarkers Market presents a wealth of exciting opportunities driven by the rising tide of chronic diseases and the increasing focus on personalized medicine. An aging population and modern lifestyle factors are contributing to a surge in autoimmune diseases and cancers, creating a significant demand for early detection tools. Autoantibody tests address this need by offering a highly specific way to identify diseases at an early stage, even before symptoms arise. This early window is crucial, allowing for prompt intervention and potentially preventing irreversible damage or improving treatment efficacy for cancers. Furthermore, autoantibody profiles can provide valuable insights into a patient's unique immune response, paving the way for personalized treatment strategies. By understanding the specific autoantibodies present, doctors can tailor treatment plans to target those molecules, potentially leading to more effective and tolerable therapies for each individual. This personalized approach to medicine holds immense promise for improving patient outcomes and reducing healthcare costs associated with managing advanced stages of diseases. Technological advancements are another key driver, with developments in protein microarrays and multiplex assays facilitating the discovery and validation of novel autoantibody biomarkers. This broader range of biomarkers has the potential to expand the reach of autoantibody-based diagnostics to a wider array of conditions. However, overcoming challenges like high development costs, variability in immune responses, and lengthy regulatory hurdles will be crucial for the market to reach its full potential. Collaboration between stakeholders, cost-effective technology advancements, and increased awareness campaigns can address these limitations and unlock the true transformative potential of autoantibody-based diagnostics, ushering in a new era of precision medicine with earlier diagnoses, improved treatment strategies, and ultimately, better patient outcomes.
AUTOANTIBODY-BASED DIAGNOSTIC BIOMARKERS MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
7.17% |
|
Segments Covered |
By Type, Application, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Abbott Laboratories, F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc., Siemens Healthineers AG, Mayo Clinic, Quest Diagnostics Incorporated, Laboratory Corporation of America Holdings, Inova Diagnostics Inc., DiaSorin S.p.A., Euroimmun AG |
Autoantibody-Based Diagnostic Biomarkers Market segmentation - By Type
-
Disease-Specific Biomarkers
-
Disease-Associated Biomarkers
Currently, Disease-Specific Biomarkers are likely the most prominent sector in the Autoantibody-Based Diagnostic Biomarkers Market. This is because they offer the advantage of a definitive diagnosis for a particular disease. Examples like anti-CCP antibodies for rheumatoid arthritis provide a clear signal for a specific condition, allowing for targeted treatment and improved patient outcomes. Disease-Associated Biomarkers, while valuable, can point to a range of related conditions, requiring further testing for a confirmed diagnosis. As the market matures and technology advances, biomarker panels combining multiple markers might emerge as a strong competitor, but for now, disease-specific biomarkers hold the lead due to their precision and clear diagnostic value.
Autoantibody-Based Diagnostic Biomarkers Market segmentation - By Application
-
Autoimmune Diseases
-
Neurodegenerative Diseases
Currently, Autoimmune Diseases are the most prominent application area within the Autoantibody-Based Diagnostic Biomarkers Market. While Neurodegenerative Diseases hold promise for future applications of autoantibody-based diagnostics, the field is still under development. More research is needed to validate the role of autoantibodies in these diseases and translate it into reliable diagnostic tests. Therefore, Autoimmune Diseases remain the dominant sector due to the stronger foundation of scientific understanding and the pressing need for improved diagnostic tools in this area
Autoantibody-Based Diagnostic Biomarkers Market segmentation - Regional Analysis
-
Asia Pacific
-
North America
-
Europe
-
South America
-
Middle East and Africa
Currently, North America is likely the most dominant region in the Autoantibody-Based Diagnostic Biomarkers Market. This dominance stems from several factors: a strong presence of established pharmaceutical and biotechnology companies driving research and development, a supportive regulatory environment for new diagnostics, and high healthcare spending. While regions like Asia Pacific are showing significant growth due to rising chronic disease prevalence and increasing healthcare investments, North America's established infrastructure and focus on innovation keep it at the forefront for now.
COVID-19 Impact Analysis on the Global Autoantibody-Based Diagnostic Biomarkers Market
The COVID-19 pandemic has had a mixed impact on the Global Autoantibody-Based Diagnostic Biomarkers Market. On the one hand, it presented challenges. Research activities and clinical trials for new autoantibody tests were potentially delayed due to resource allocation towards COVID-19 diagnostics and patient care. Additionally, with healthcare systems stretched thin, routine diagnoses for some autoimmune diseases might have been postponed, impacting the immediate market growth. However, the pandemic also highlighted the need for efficient and specific diagnostic tools. This could lead to increased long-term investments in biomarker research, including autoantibody-based tests. Furthermore, a growing emphasis on early disease detection and personalized medicine post-pandemic might benefit the autoantibody-based diagnostics market as these tests offer significant advantages in these areas. Overall, the COVID-19 pandemic's net impact on the market remains to be seen, but it has the potential to be a double-edged sword, presenting both temporary hurdles and long-term growth opportunities.
Latest trends/Developments
The Autoantibody-Based Diagnostic Biomarkers Market is experiencing exciting advancements driven by a focus on early disease detection and personalized medicine. One key trend is the development of biomarker panels. These combine multiple autoantibody markers to improve diagnostic accuracy and potentially identify disease sub-types. This approach holds promise for conditions like autoimmune diseases where a single marker might not be sufficient for a definitive diagnosis. Technological advancements are another area of active development. New platforms like multiplex assays allow researchers to analyze a wider range of autoantibodies simultaneously, facilitating biomarker discovery and test development. Artificial intelligence is also playing an increasing role, with algorithms being used to analyze complex autoantibody data and improve diagnostic accuracy. Furthermore, the focus is shifting towards earlier disease detection. Autoantibody tests are being explored to identify individuals at risk of developing autoimmune diseases before symptoms even appear. This early intervention window has the potential to significantly improve patient outcomes and potentially prevent irreversible damage. Overall, the Autoantibody-Based Diagnostic Biomarkers Market is a rapidly evolving field with the potential to revolutionize diagnostics by offering a more precise and personalized approach to disease detection and management.
Key Players:
-
Abbott Laboratories
-
F. Hoffmann-La Roche Ltd.
-
Thermo Fisher Scientific Inc.
-
Siemens Healthineers AG
-
Mayo Clinic
-
Quest Diagnostics Incorporated
-
Laboratory Corporation of America Holdings
-
Inova Diagnostics Inc.
-
DiaSorin S.p.A.
-
Euroimmun AG
Chapter 1. Autoantibody-Based Diagnostic Biomarkers Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. Autoantibody-Based Diagnostic Biomarkers Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. Autoantibody-Based Diagnostic Biomarkers Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. Autoantibody-Based Diagnostic Biomarkers Market - Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. Autoantibody-Based Diagnostic Biomarkers Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. Autoantibody-Based Diagnostic Biomarkers Market – By Type
6.1 Introduction/Key Findings
6.2 Disease-Specific Biomarkers
6.3 Disease-Associated Biomarkers
6.4 Y-O-Y Growth trend Analysis By Type
6.5 Absolute $ Opportunity Analysis By Type, 2024-2030
Chapter 7. Autoantibody-Based Diagnostic Biomarkers Market – By Application
7.1 Introduction/Key Findings
7.2 Autoimmune Diseases
7.3 Neurodegenerative Diseases
7.4 Y-O-Y Growth trend Analysis By Application
7.5 Absolute $ Opportunity Analysis By Application, 2024-2030
Chapter 8. Autoantibody-Based Diagnostic Biomarkers Market , By Geography – Market Size, Forecast, Trends & Insights
8.1 North America
8.1.1 By Country
8.1.1.1 U.S.A.
8.1.1.2 Canada
8.1.1.3 Mexico
8.1.2 By Type
8.1.3 By Application
8.1.4 Countries & Segments - Market Attractiveness Analysis
8.2 Europe
8.2.1 By Country
8.2.1.1 U.K
8.2.1.2 Germany
8.2.1.3 France
8.2.1.4 Italy
8.2.1.5 Spain
8.2.1.6 Rest of Europe
8.2.2 By Type
8.2.3 By Application
8.2.4 Countries & Segments - Market Attractiveness Analysis
8.3 Asia Pacific
8.3.1 By Country
8.3.1.1 China
8.3.1.2 Japan
8.3.1.3 South Korea
8.3.1.4 India
8.3.1.5 Australia & New Zealand
8.3.1.6 Rest of Asia-Pacific
8.3.2 By Type
8.3.3 By Application
8.3.4 Countries & Segments - Market Attractiveness Analysis
8.4 South America
8.4.1 By Country
8.4.1.1 Brazil
8.4.1.2 Argentina
8.4.1.3 Colombia
8.4.1.4 Chile
8.4.1.5 Rest of South America
8.4.2 By Type
8.4.3 By Application
8.4.4 Countries & Segments - Market Attractiveness Analysis
8.5 Middle East & Africa
8.5.1 By Country
8.5.1.1 United Arab Emirates (UAE)
8.5.1.2 Saudi Arabia
8.5.1.3 Qatar
8.5.1.4 Israel
8.5.1.5 South Africa
8.5.1.6 Nigeria
8.5.1.7 Kenya
8.5.1.8 Egypt
8.5.1.9 Rest of MEA
8.5.2 By Type
8.5.3 By Application
8.5.4 Countries & Segments - Market Attractiveness Analysis
Chapter 9. Autoantibody-Based Diagnostic Biomarkers Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
9.1 Abbott Laboratories
9.2 F. Hoffmann-La Roche Ltd.
9.3 Thermo Fisher Scientific Inc.
9.4 Siemens Healthineers AG
9.5 Mayo Clinic
9.6 Quest Diagnostics Incorporated
9.7 Laboratory Corporation of America Holdings
9.8 Inova Diagnostics Inc.
9.9 DiaSorin S.p.A.
9.10 Euroimmun AG
Download Sample
Choose License Type
2500
4250
5250
6900
Frequently Asked Questions
The Global Autoantibody-Based Diagnostic Biomarkers Market was valued at USD 5.32 billion in 2023 and will grow at a CAGR of 7.17% from 2024 to 2030. The market is expected to reach USD 8.64 billion by 2030.
Rising Prevalence of Chronic Diseases, Improved Diagnostic Accuracy, Increased Focus on Personalized Medicine these are the reasons which is driving the market.
Based on Application it is divided into two segments – Disease-Specific Biomarkers, Disease-Associated Biomarkers.
North America is the most dominant region for the luxury vehicle Market.
Abbott Laboratories, F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc., Siemens Healthineers AG, Mayo Clinic.



