Chapter 1. ANAESTHESIA AND RESPIRATORY DEVICES MARKET– Scope & Methodology
1.1. Market Segmentation
1.2. Assumptions
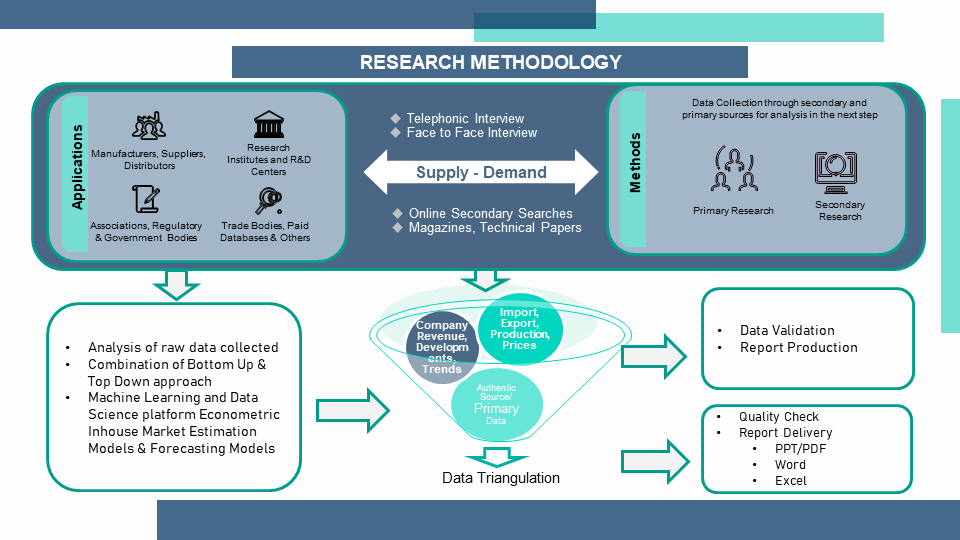
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2. ANAESTHESIA AND RESPIRATORY DEVICES MARKET– Executive Summary
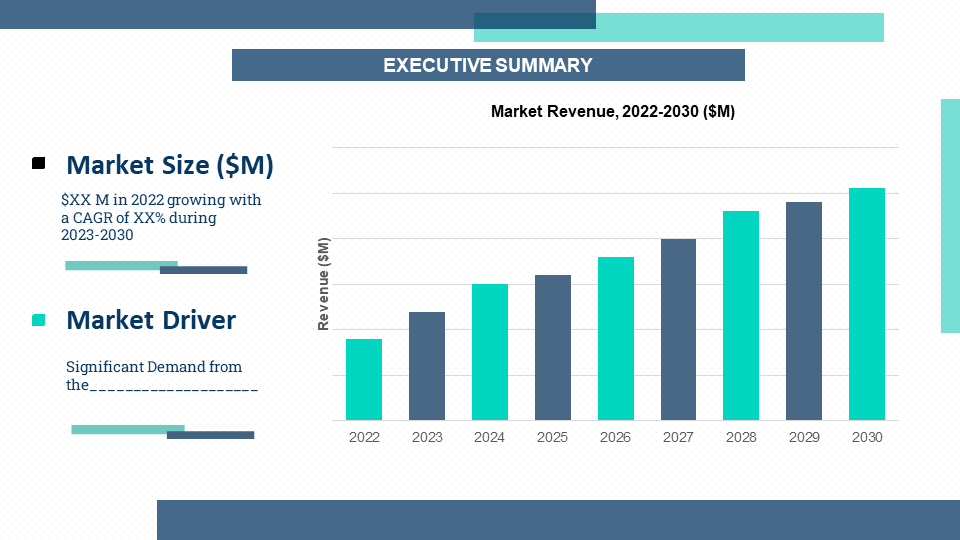
2.1. Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-81 Impact Analysis
2.3.1. Impact during 2023 – 2030
2.3.2. Impact on Supply – Demand
Chapter 3. ANAESTHESIA AND RESPIRATORY DEVICES MARKET– Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4. ANAESTHESIA AND RESPIRATORY DEVICES MARKET- Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatory Scenario - By Region
4.3 Customer Analysis
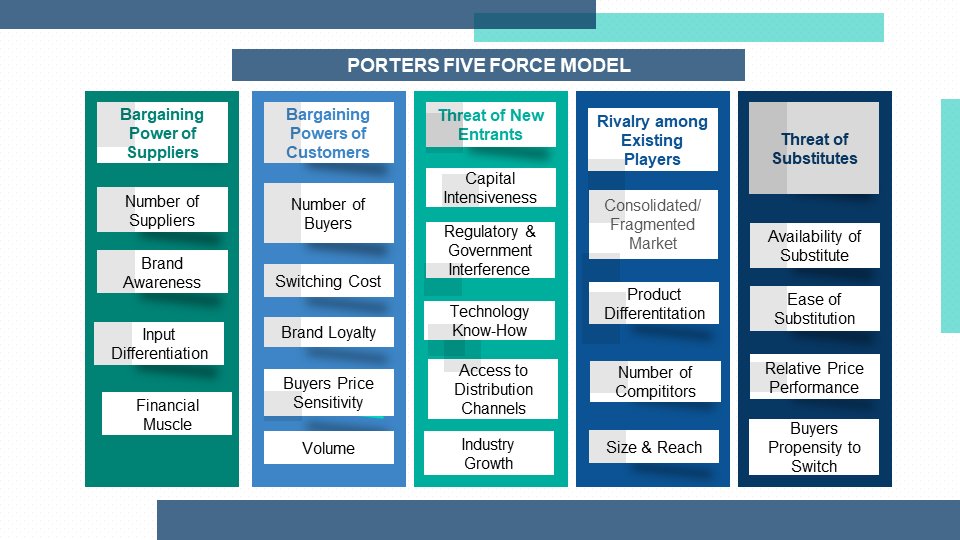
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. ANAESTHESIA AND RESPIRATORY DEVICES MARKET- Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
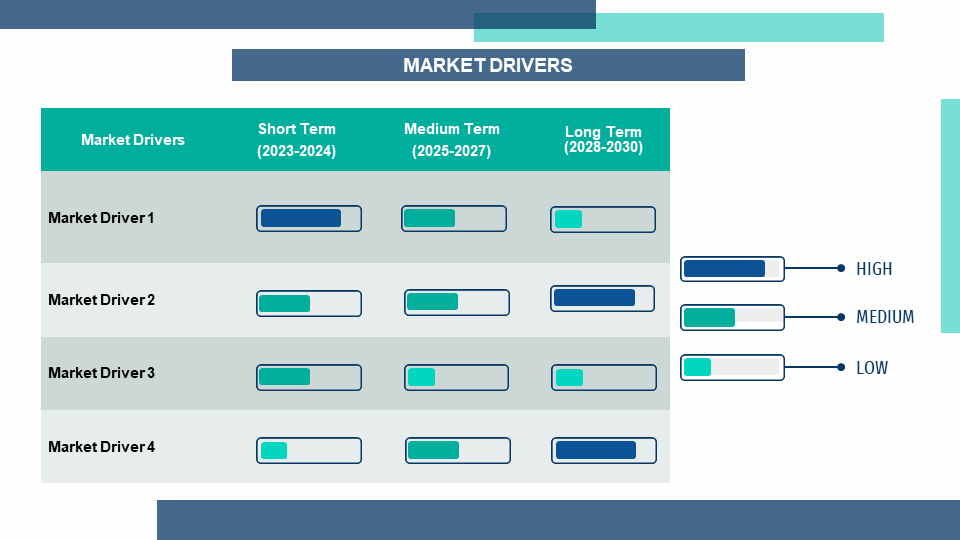
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6. ANAESTHESIA AND RESPIRATORY DEVICES MARKET– By Product
6.1. Respiratory Devices
6.1.1. Respiratory Equipment
6.1.1.1. Humidifiers
6.1.1.1.1. Heat Humidifiers
6.1.1.1.2. Heated Wire Breathing Circuits
6.1.1.1.3. Heat Exchangers
6.1.1.1.4. Pass Over Humidifiers
6.1.1.2 Nebulizers
6.1.1.2.1. Pneumatic Nebulizers
6.1.1.2.2. Mesh Nebulizers
6.1.1.2.3. Ultrasonic Nebulizers
6.1.1.3. Oxygen Concentrator
6.1.1.3.1. Fixed Oxygen Concentrators
6.1.1.3.2. Portable Oxygen Concentrators
6.1.1.4. Positive Airway Pressure Devices
6.1.1.4.1. Bilevel & automatic positive airway pressure devices
6.1.1.4.2. Continuous positive airway pressure devices
6.1.1.5. Reusable Resuscitators
6.1.1.6. Ventilators
6.1.1.6.1. Adult Ventilators
6.1.1.6.2. Neonatal Ventilators
6.1.1.7. Respiratory Inhalers
6.1.1.7.1. Dry Powdered Inhalers
6.1.1.7.2. Metered Dose Inhaler
6.1.1.8. Respiratory Disposables
6.1.1.8.1. Disposable Oxygen Masks
6.1.1.8.2. Resuscitators
6.1.1.8.3. Tracheostomy Tubes
6.1.1.8.4. Oxygen Cannula
6.1.1.9. Respiratory Measurement Devices
6.1.1.9.1. Pulse Oximetry systems
6.1.1.9.2. Capnographs
6.1.1.9.3. Spirometers
6.1.1.9.4. Peak Flowmeters
6.2. Anesthesia Devices
6.2.1. Anesthesia Machines
6.2.1.1. Anesthesia Workstation
6.2.1.2. Anesthesia delivery machines
6.2.1.2.1. Portable
6.2.1.2.2. Standalone
6.2.1.3. Anesthesia Ventilators
6.2.1.4. Anesthesia Monitors
6.2.2. Anesthesia Disposables
6.2.2.1. Anesthesia Masks
6.2.2.2. Anesthesia Accessories
Chapter 7. ANAESTHESIA AND RESPIRATORY DEVICES MARKET– By Region
7.1. North America
7.2. Europe
7.3. The Asia Pacific
7.4. Latin America
7.5. Middle-East and Africa
Chapter 8. ANAESTHESIA AND RESPIRATORY DEVICES MARKET – Company Profiles – (Overview, Product Portfolio, Financials, Developments)
8.1. Company 1
8.2. Company 2
8.3. Company 3
8.4. Company 4
8.5. Company 5
8.6. Company 6
8.7. Company 7
8.8. Company 8
8.9. Company 9
8.10. Company 10
Download Sample
Choose License Type
2500
4250
5250
6900



