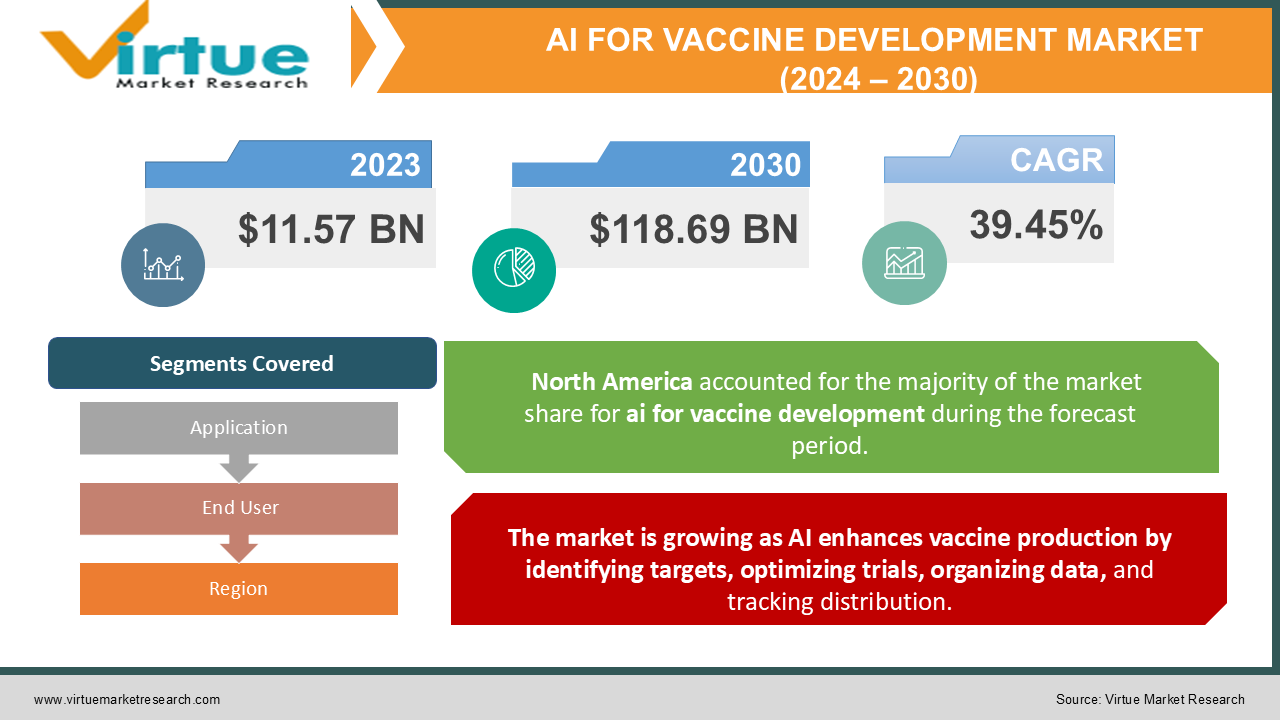
AI for Vaccine Development Market Size (2024 –2030)
The global AI for Vaccine Development Market was valued at USD 11.57 billion in 2023 and is estimated to reach USD 118.69 billion by 2030 growing at a CAGR of 39.45% during the forecast period 2024-2030.
The increasing number of infections is driving up the global market for AI in Vaccine Development. Artificial intelligence, or AI, is being used by many businesses to help create vaccines more quickly and effectively. AI is helpful in many ways, including identifying the best targets for vaccines, testing them in laboratories, arranging data for scientists, and monitoring vaccine distribution. The process of developing new medications and vaccines is typically expensive and time-consuming. However, AI can expedite this process by analyzing multiple variables concurrently and forecasting which targets are most suitable for vaccine development. Additionally, it can aid in the comprehension of viral mechanisms, a crucial aspect of developing vaccines.
AI is also useful for creating medications by analyzing their molecular makeup. It can help repurpose current medications and forecast which targets might be most beneficial for novel ones. AI is being used by pharmaceutical companies to expedite and simplify the process of developing new drugs. It can forecast a medication's potential effectiveness as well as any potential side effects. AI also aids in the management of all the data required to produce and market pharmaceuticals.
Key Market Insights:
- North America accounts for nearly 30% of the global AI for vaccine development market share, attributed to the strong presence of leading pharmaceutical companies, advanced research capabilities, and substantial investments in AI and vaccine development.
- Machine learning holds a market share of over 35% in the AI for vaccine development market, owing to its ability to analyze vast amounts of data, identify patterns, and predict potential vaccine candidates.
- Drug discovery and development contributes to around 50% of the overall AI for the vaccine development market, driven by the use of AI in various stages, including target identification, lead optimization, and clinical trial design.
- The academic and research institutes segment is expected to grow at a rate of around 10% annually, driven by the increasing collaboration between academia, research organizations, and pharmaceutical companies to leverage AI in vaccine research and development.
Global AI for Vaccine Development Market Drivers:
The market is growing as AI enhances vaccine production by identifying targets, optimizing trials, organizing data, and tracking distribution.
Drug testing is being expedited by artificial intelligence (AI) technology, as the traditional approach is laborious. Scientists can investigate the virus and gain a better understanding of its structure with AI. Their ability to anticipate which viral components may elicit an immune response is crucial for the development of vaccines. AI also aids in the analysis of the virus's evolutionary history, which is particularly useful for viruses like COVID-19 that have multiple variants.
Scientists need a lot of information to comprehend the virus and how our immune system reacts to it in order to develop a vaccine. Massive data sets can be combed through by AI algorithms to identify the best candidates for vaccine targets. AI can assist scientists in determining the appropriate dosage for a vaccine, testing it in a lab setting, and forecasting potential immune system reactions once they have a list of targets. It can even aid in determining the best locations for clinical trials involving human vaccine testing. For instance, MIT researchers developed a machine learning-powered model named COVID-19 that tracks people's health and behavior and provides real-time information about the epidemic. Not only can AI be used to create vaccines, but it can also be used to distribute vaccines, monitor vaccination rates, and offer vaccination advice.
An increase in the prevalence of infectious diseases, significant R&D efforts, and the application of AI in healthcare by numerous pharmaceutical and biotechnology companies globally are driving the market for AI in vaccine development.
Worldwide, infectious diseases continue to be a major issue, particularly in developing nations where they claim millions of lives annually. Since the advent of mathematical techniques, scientists have become increasingly adept at understanding various diseases, forecasting outbreaks, and identifying potential targets for novel drugs. The ability of artificial intelligence, or AI, to distinguish between various forms of cancer from photos has garnered a lot of interest. Moreover, an increasing number of biotech and pharmaceutical firms are utilizing AI to produce medications and vaccines more quickly.
AI for Vaccine Development Market Challenges and Restraints:
Healthcare data collection and utilization are highly regulated due to patient privacy laws and other significant issues. Hospitals typically employ outside specialists to create their AI algorithms. This implies that they may use patient data from individuals who are not precisely like the patients at that hospital. Data sharing between hospitals is complicated by issues with data security, integrating disparate systems, and maintaining patient confidentiality. This further complicates the task of verifying the accuracy of AI algorithms developed within hospitals. Furthermore, it's possible that AI algorithms aren't always fair, which could result in unfair patient treatment. Hospitals require powerful computers with specialized hardware and fast internet in order to use AI for drug discovery. These computers come in handy for tasks like rapidly and effectively testing a large number of potential drugs. However, the speed and cost of these computers can determine how effective these drug discovery methods are.
AI for Vaccine Development Market Opportunities:
The market for AI in vaccine development offers enormous opportunities. First of all, AI can expedite the entire vaccine development process, reducing the time it takes to identify viable candidates and prepare them for use. Then, using AI, vaccines could be made more effectively and safely by being genetically customized for each individual. Modifying the formulas of vaccines and forecasting our bodies' reactions to them, can also enhance their effectiveness. AI has the potential to save time and money by identifying medications that are currently on the market that could be turned into vaccines. Furthermore, AI can forecast the potential onset of diseases, allowing us to prepare vaccines ahead of time. It's also excellent at identifying emerging threats and tracking disease trends, which speeds up our response time. Artificial intelligence has the potential to expedite and reduce the cost of vaccine production, ensuring sufficient supply for all those in need. Governments, businesses, and researchers can maximize these opportunities and enhance everyone's access to global health by collaborating.
AI FOR VACCINE DEVELOPMENT MARKET REPORT COVERAGE:
|
REPORT METRIC |
DETAILS |
|
Market Size Available |
2023 - 2030 |
|
Base Year |
2023 |
|
Forecast Period |
2024 - 2030 |
|
CAGR |
39.45% |
|
Segments Covered |
By Application, End User, and Region |
|
Various Analyses Covered |
Global, Regional & Country Level Analysis, Segment-Level Analysis, DROC, PESTLE Analysis, Porter’s Five Forces Analysis, Competitive Landscape, Analyst Overview on Investment Opportunities |
|
Regional Scope |
North America, Europe, APAC, Latin America, Middle East & Africa |
|
Key Companies Profiled |
Benevolent AI, Pfizer, Evaxion Biotech, AstraZeneca, Eli Lilly, Moderna, Novartis, Baidu, Exscientia, Roche |
AI for Vaccine Development Market – By Application
-
Vaccine Exploration/Preclinical
-
Vaccine Clinical Developments and Trials
-
Vaccine Manufacturing and QC
Using AI, researchers can quickly identify potential treatments in the early stages of drug development by sorting through massive amounts of data, a process that would take years to complete by hand. They can also match treatments to individual patients' genes and immune responses by using AI to analyze complex genetic data. A vaccine is tested after it is created to ensure that it is both safe and effective. Before it can be mass-produced, regulators must give their approval. AI and sensors are being used by businesses to streamline the production and delivery of vaccines, cutting down on waste and guaranteeing supply for all those in need. To ensure that vaccines are safe, effective, and scalable, each of these steps is critical. AI is accelerating the process, which has traditionally been costly and time-consuming. Growing efforts by large pharmaceutical companies, continuous research, and increased government funding are propelling the market for artificial intelligence in vaccine development.
AI for Vaccine Development Market – By End User
-
Pharmaceutical & biotechnology companies
-
Contract Research Organization
-
Research centers
With over 42% of the total revenue in 2023, pharmaceutical and biopharmaceutical companies were the dominant players in the AI vaccine development market. They had the infrastructure for developing vaccines already, so they jumped right into COVID-19 research. Their substantial contributions accelerated the development of COVID-19 vaccines. These businesses are now working together to produce vaccines more quickly. By developing online resources to aid in the speedy development of vaccines and examining the data they generate, research institutes are also contributing to the cause.
AI for Vaccine Development Market - By Region
-
North America
-
Europe
-
Asia-Pacific
-
Rest of the World
In terms of applying AI to vaccine development in 2023, North America took the lead, followed by Europe and Asia-Pacific. This is a result of these areas' rapid adoption of new technologies. The increase in North America is a result of increased funding, research, and major players in the field, in addition to a high number of COVID-19 cases and vaccinations. This was enhanced by the US government's Operation Warp Speed, which assisted in the development, manufacturing, and distribution of COVID-19 vaccines. Along with leading pharmaceutical companies like Pfizer and Johnson & Johnson, which heavily invest in research, this region is home to numerous large AI companies and startups. Places like China, India, and the Middle East present significant growth opportunities for AI vaccine development companies. This is due to the fact that these nations have growing populations, improved healthcare systems, and rising income levels. It works particularly well for businesses that are struggling in more established markets with slower growth, eroding patents, and stricter regulations.
COVID-19 Impact on the global AI for Vaccine Development Market:
Numerous teams around the world raced to develop vaccines during the Covid-19 pandemic. For instance, China had a significant outbreak very early on. Some companies, like Pfizer and Baidu, accelerated the development of vaccines by using AI technologies. Additionally, Google's Deepmind employed AI to forecast the structures of some COVID-19-related proteins, which may be useful for upcoming studies and medical interventions. AI has been incredibly useful in the search for possible anti-COVID-19 medications by examining various datasets. It has been particularly helpful in identifying the disease and determining which medications may be most effective in treating it. The lack of high-quality data, on occasion, presents a problem for researchers and may reduce the accuracy of their predictions. Nevertheless, organizations all over the world are utilizing AI for testing more and more, which is fueling the growth of the AI for Vaccine Development market.
Latest Trend/Development:
Artificial intelligence (AI) is creating quite a stir in the vaccine development industry with some fascinating new developments. Firstly, by analyzing an individual's genes and immune system, AI is assisting in the development of personalized vaccines that are more effective for each person. Predictive modeling is another approach that uses AI to determine which vaccines might be most effective, resulting in a quicker and less expensive process. AI is also being used in the design of new vaccine components that enable us to fight diseases that were previously unfightable. Vaccines are becoming easier to store and more stable as a result. Additionally, AI is collaborating with other technologies to create vaccine combos that can combat several diseases at once. Furthermore, in order for us to act swiftly, monitoring diseases in real-time is just as important as developing vaccines. All of this is made possible by AI processing massive amounts of data from various disciplines, including epidemiology and genetics. More than ever, businesses and researchers are collaborating, and everyone is considering the ethical applications of AI. Finally, authorities are enacting regulations to maintain safety and equity as they begin to recognize the potential of AI in vaccines. We're getting closer to creating vaccines that function better, faster, and for everyone with AI's assistance.
Key Players:
-
Benevolent AI
-
Pfizer
-
Evaxion Biotech
-
AstraZeneca
-
Eli Lilly
-
Moderna
-
Novartis
-
Baidu
-
Exscientia
-
Roche
Market News:
-
AstraZeneca and BenevolentAI agreed to intensify their collaboration in January 2022 to use artificial intelligence (AI) to discover novel treatments for lupus and heart failure.
-
Exscientia and Sanofi collaborated to use AI to develop up to 15 novel therapeutic candidates for immune system disorders and cancer in January 2022.
-
GlaxoSmithKline (GSK) and PathAI partnered in April 2022 to use PathAI's technology to aid in the development of cancer and liver disease treatments.
-
Additionally, in April 2022, a collaboration between Japan's NEC Group and CEPI began to develop a vaccine capable of defending against a variety of coronaviruses. To develop this vaccine, NEC OncoImmunity AS will receive up to $4.8 million in funding from CEPI.
Chapter 1. AI for Vaccine Development Market – Scope & Methodology
1.1 Market Segmentation
1.2 Scope, Assumptions & Limitations
1.3 Research Methodology
1.4 Primary Sources
1.5 Secondary Sources
Chapter 2. AI for Vaccine Development Market – Executive Summary
2.1 Market Size & Forecast – (2024 – 2030) ($M/$Bn)
2.2 Key Trends & Insights
2.2.1 Demand Side
2.2.2 Supply Side
2.3 Attractive Investment Propositions
2.4 COVID-19 Impact Analysis
Chapter 3. AI for Vaccine Development Market – Competition Scenario
3.1 Market Share Analysis & Company Benchmarking
3.2 Competitive Strategy & Development Scenario
3.3 Competitive Pricing Analysis
3.4 Supplier-Distributor Analysis
Chapter 4. AI for Vaccine Development Market - Entry Scenario
4.1 Regulatory Scenario
4.2 Case Studies – Key Start-ups
4.3 Customer Analysis
4.4 PESTLE Analysis
4.5 Porters Five Force Model
4.5.1 Bargaining Power of Suppliers
4.5.2 Bargaining Powers of Customers
4.5.3 Threat of New Entrants
4.5.4 Rivalry among Existing Players
4.5.5 Threat of Substitutes
Chapter 5. AI for Vaccine Development Market – Landscape
5.1 Value Chain Analysis – Key Stakeholders Impact Analysis
5.2 Market Drivers
5.3 Market Restraints/Challenges
5.4 Market Opportunities
Chapter 6. AI for Vaccine Development Market – By End User
6.1 Introduction/Key Findings
6.2 Pharmaceutical & biotechnology companies
6.3 Contract Research Organization
6.4 Research centers
6.5 Y-O-Y Growth trend Analysis By End User
6.6 Absolute $ Opportunity Analysis By End User, 2024-2030
Chapter 7. AI for Vaccine Development Market – By Application
7.1 Introduction/Key Findings
7.2 Vaccine Exploration/Preclinical
7.3 Vaccine Clinical Developments and Trials
7.4 Vaccine Manufacturing and QC
7.5 Y-O-Y Growth trend Analysis By Application
7.6 Absolute $ Opportunity Analysis By Application, 2024-2030
Chapter 8. AI for Vaccine Development Market , By Geography – Market Size, Forecast, Trends & Insights
8.1 North America
8.1.1 By Country
8.1.1.1 U.S.A.
8.1.1.2 Canada
8.1.1.3 Mexico
8.1.2 By End User
8.1.3 By Application
8.1.4 Countries & Segments - Market Attractiveness Analysis
8.2 Europe
8.2.1 By Country
8.2.1.1 U.K
8.2.1.2 Germany
8.2.1.3 France
8.2.1.4 Italy
8.2.1.5 Spain
8.2.1.6 Rest of Europe
8.2.2 By End User
8.2.3 By Application
8.2.4 Countries & Segments - Market Attractiveness Analysis
8.3 Asia Pacific
8.3.1 By Country
8.3.1.1 China
8.3.1.2 Japan
8.3.1.3 South Korea
8.3.1.4 India
8.3.1.5 Australia & New Zealand
8.3.1.6 Rest of Asia-Pacific
8.3.2 By End User
8.3.3 By Application
8.3.4 Countries & Segments - Market Attractiveness Analysis
8.4 South America
8.4.1 By Country
8.4.1.1 Brazil
8.4.1.2 Argentina
8.4.1.3 Colombia
8.4.1.4 Chile
8.4.1.5 Rest of South America
8.4.2 By End User
8.4.3 By Application
8.4.4 Countries & Segments - Market Attractiveness Analysis
8.5 Middle East & Africa
8.5.1 By Country
8.5.1.1 United Arab Emirates (UAE)
8.5.1.2 Saudi Arabia
8.5.1.3 Qatar
8.5.1.4 Israel
8.5.1.5 South Africa
8.5.1.6 Nigeria
8.5.1.7 Kenya
8.5.1.8 Egypt
8.5.1.9 Rest of MEA
8.5.2 By End User
8.5.3 By Application
8.5.4 Countries & Segments - Market Attractiveness Analysis
Chapter 9. AI for Vaccine Development Market – Company Profiles – (Overview, Product Portfolio, Financials, Strategies & Developments)
9.1 Benevolent AI
9.2 Pfizer
9.3 Evaxion Biotech
9.4 AstraZeneca
9.5 Eli Lilly
9.6 Moderna
9.7 Novartis
9.8 Baidu
9.9 Exscientia
9.10 Roche
Download Sample
Choose License Type
2500
4250
5250
6900
Related Reports
Frequently Asked Questions
In 2023, the global AI for Vaccine Development Market was valued at $11.57 billion and is estimated to reach $118.69 billion by 2030.
Key players in the AI for Vaccine Development Market are Benevolent AI, Pfizer, Evaxion Biotech, AstraZeneca, Eli Lilly, Moderna, Novartis, Baidu, Exscientia, Roche, VantAI, Sanofi, Cyclica, PathAI, Atomwise, BioSymetrics.
North America is predicted to dominate the global market due to the existence of a significant number of pharmaceutical and biotechnology businesses, research institutions, and government support for vaccine development.
The market is growing as a result of artificial intelligence's (AI) numerous advantages in the production of vaccines, such as its capacity to identify vaccine targets, streamline clinical trials and preclinical testing, arrange data for researchers, monitor vaccine distribution, and more.
Asia-Pacific is the fastest-growing region in the global AI for Vaccine Development Market.



