Chapter 1. ACTIVE IMPLANTABLE MEDICAL DEVICES MARKET – Scope & Methodology
1.1. Market Segmentation
1.2. Assumptions
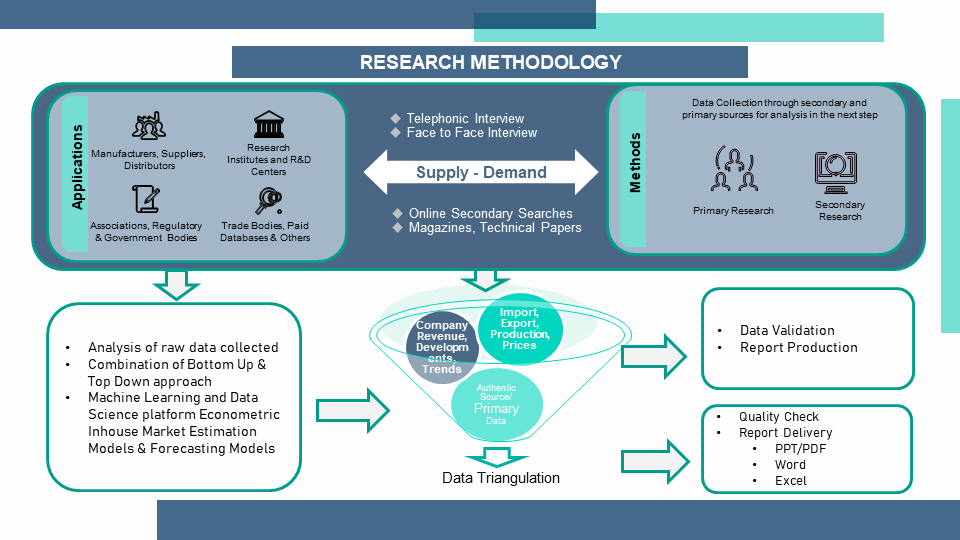
1.3. Research Methodology
1.4. Primary Sources
1.5. Secondary Sources
Chapter 2. ACTIVE IMPLANTABLE MEDICAL DEVICES MARKET – Executive Summary
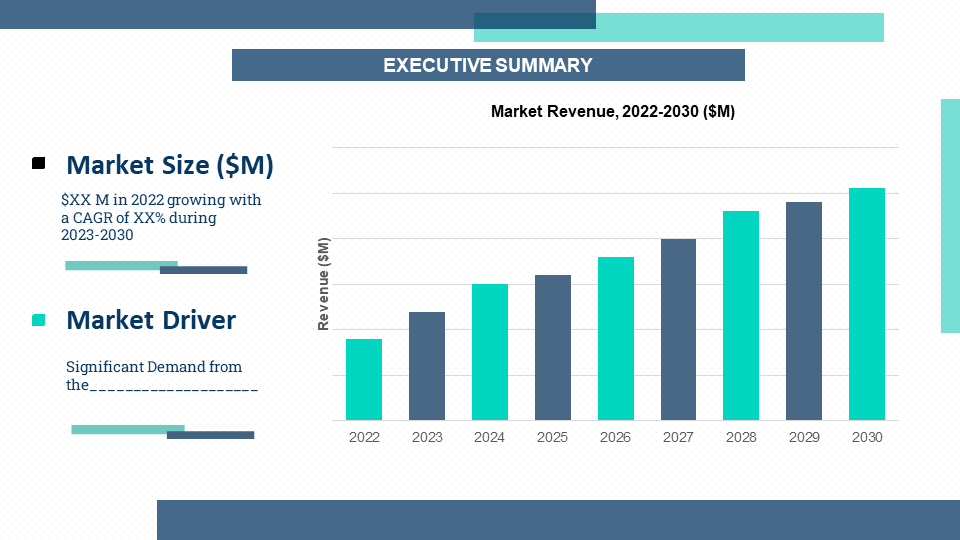
2.1. Market Size & Forecast – (2023 – 2030) ($M/$Bn)
2.2. Key Trends & Insights
2.3. COVID-19 Impact Analysis
2.3.1. Impact during 2023 - 2030
2.3.2. Impact on Supply – Demand
Chapter 3. ACTIVE IMPLANTABLE MEDICAL DEVICES MARKET – Competition Scenario
3.1. Market Share Analysis
3.2. Product Benchmarking
3.3. Competitive Strategy & Development Scenario
3.4. Competitive Pricing Analysis
3.5. Supplier - Distributor Analysis
Chapter 4. ACTIVE IMPLANTABLE MEDICAL DEVICES MARKET - Entry Scenario
4.1. Case Studies – Start-up/Thriving Companies
4.2. Regulatorycenario - By Region
4.3 Customer Analysis
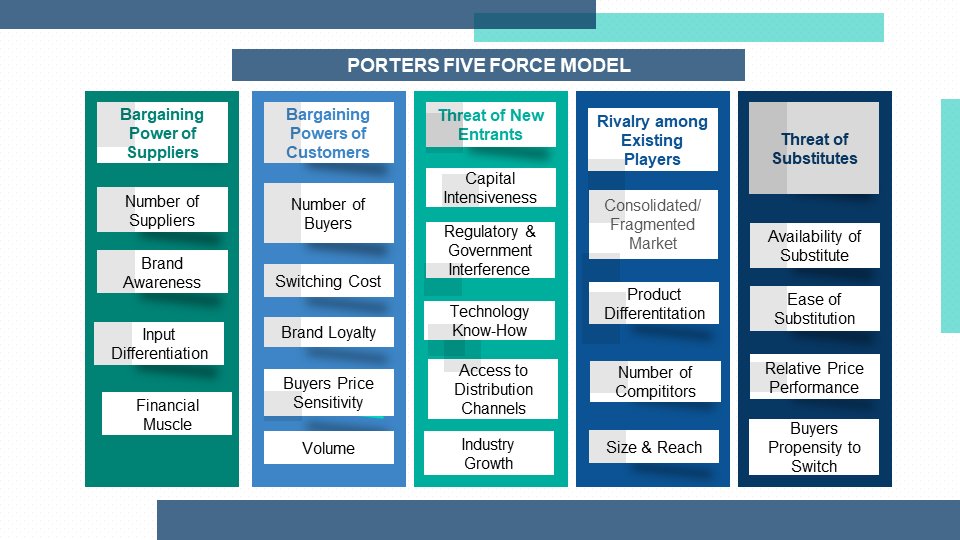
4.4. Porter's Five Force Model
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Powers of Customers
4.4.3. Threat of New Entrants
4.4.4. Rivalry among Existing Players
4.4.5. Threat of Substitutes
Chapter 5. ACTIVE IMPLANTABLE MEDICAL DEVICES MARKET - Landscape
5.1. Value Chain Analysis – Key Stakeholders Impact Analysis
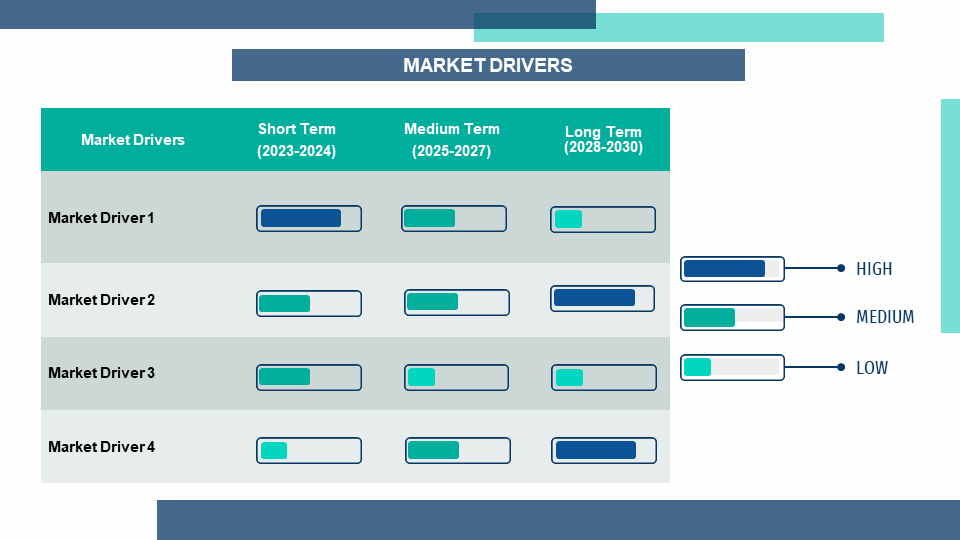
5.2. Market Drivers
5.3. Market Restraints/Challenges
5.4. Market Opportunities
Chapter 6. ACTIVE IMPLANTABLE MEDICAL DEVICES MARKET – By Product Type
6.1. Implantable Cardioverter Defibrillators
6.2. Transvenous Implantable Cardioverter Defibrillators
6.3. Biventricular Implantable Cardioverter Defibrillators/Cardiac Resynchronization Therapy Defibrillators
6.4. Dual-Chamber Implantable Cardioverter Defibrillators
6.5. Single-Chamber Implantable Cardioverter Defibrillators
6.6. Subcutaneous Implantable Cardioverter Defibrillators
6.7. Ventricular Assist Devices
6.8. Implantable Heart Monitors/Insertable Loop Recorders
6.9. Neurostimulators
6.10. Spinal Cord Stimulators
6.11. Deep Brain Stimulators
6.12. Sacral Nerve Stimulators
6.13. Vagus Nerve Stimulators
6.14. Gastric Electrical Stimulators
6.15. Implantable Hearing Devices
6.16. Active Hearing Implants
6.17. Non-active/Passive Hearing Implants
Chapter 7. ACTIVE IMPLANTABLE MEDICAL DEVICES MARKET – By Region
7.1. North America
7.2. Europe
7.3. Asia-P2acific
7.4. Latin America
7.5. The Middle East
7.6. Africa
Chapter 8. ACTIVE IMPLANTABLE MEDICAL DEVICES MARKET – By Companies
8.1. Companies 1
8.2. Companies 2
8.3. Companies 3
8.4. Companies 4
8.5. Companies 5
8.6. Companies 6
8.7. Companies 7
8.8. Companies 8
8.9. Companies 9
8.10. Companies 10
Download Sample
Choose License Type
2500
4250
5250
6900



